
In a dry cell, what acts as a negative electrode?
A. Zinc
B. Graphite
C. Ammonium chloride
D. Manganese dioxide
Answer
592.2k+ views
Hint: The dry cell is also called Leclanche cell and comes under the category of primary cell as it is not rechargeable. The negative electrode is a metal which is principally used in the galvanization of iron.
Complete step by step answer:
First, let us discuss the dry cell, it consists of two types i.e. primary cell, and secondary cell. The zinc-carbon cell is considered to be an example of a primary cell.
If we talk about the primary cells, that are neither reusable, nor it can be recharged. When there is completion of chemical reactions, it will not produce electricity again.
Talking about the zinc-carbon primary cell, it is a kind of metal container having the metal electrode covered by electrolyte paste. The zinc-carbon cell, as mentioned, consists of a zinc electrode, and a carbon rod. Thus, we can say a metal container has two electrodes, one acts as anode, and another as cathode.
The diagrammatic representation is shown below-
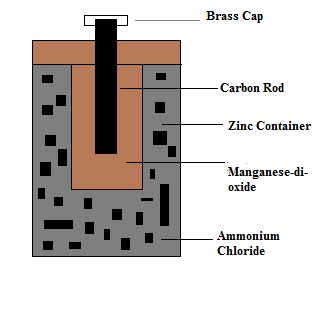
The zinc electrode will act as an anode, as it undergoes oxidation reaction; whereas inert carbon rod acts as cathode, or we can say positive electrode.
Further talking about the metal container, there is presence of manganese dioxide, as it surrounds the electrode, which is called electrolyte paste and it has low moisture. ammonium chloride is also considered as electrolyte paste.
Thus, in the last we can conclude that in the dry cell, zinc acts as a negative electrode, or anode.
Hence, the correct option is (A).
Note:
Another modified example of dry-cell is the alkaline battery. It is almost the same to the zinc-carbon batteries except with the fact that the electrolyte used is KOH (potassium hydroxide) instead of ammonium chloride. The potential of the dry cell reaction is 1.5V.
Complete step by step answer:
First, let us discuss the dry cell, it consists of two types i.e. primary cell, and secondary cell. The zinc-carbon cell is considered to be an example of a primary cell.
If we talk about the primary cells, that are neither reusable, nor it can be recharged. When there is completion of chemical reactions, it will not produce electricity again.
Talking about the zinc-carbon primary cell, it is a kind of metal container having the metal electrode covered by electrolyte paste. The zinc-carbon cell, as mentioned, consists of a zinc electrode, and a carbon rod. Thus, we can say a metal container has two electrodes, one acts as anode, and another as cathode.
The diagrammatic representation is shown below-

The zinc electrode will act as an anode, as it undergoes oxidation reaction; whereas inert carbon rod acts as cathode, or we can say positive electrode.
Further talking about the metal container, there is presence of manganese dioxide, as it surrounds the electrode, which is called electrolyte paste and it has low moisture. ammonium chloride is also considered as electrolyte paste.
Thus, in the last we can conclude that in the dry cell, zinc acts as a negative electrode, or anode.
Hence, the correct option is (A).
Note:
Another modified example of dry-cell is the alkaline battery. It is almost the same to the zinc-carbon batteries except with the fact that the electrolyte used is KOH (potassium hydroxide) instead of ammonium chloride. The potential of the dry cell reaction is 1.5V.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




