
In a Daniel cell, if A (\[{E^ \circ }\]=-0.76C) and B (\[{E^ \circ }\]=-2.36V) half cells are taken then:
A. B acts as anode
B. A acts as anode
C. B acts as cathode
D. Cannot be predicted
Answer
569.7k+ views
Hint: The essential structure of the Daniel cell must be known. The chemical reactions occurring at the respective anode and cathode result in the production of the electric current. The salt bridge plays a crucial role by allowing anions to the anode compartment and cations to the cathode compartment.
Complete answer:
We have to remember that a Daniel cell may be a galvanic cell which converts the chemical energy into the electrical energy. It consists of two half cells. One half cell is a beaker containing a strip of metallic zinc dipped in 1M aqueous zinc sulfate solution. The second half cell consists of a beaker having a metallic strip of copper immersed in 1M aqueous copper sulfate solution.
The construction of the Daniel cell includes a copper container which is crammed with the diluted sulphuric acid immersed within the copper container. The two different metals are connected to each other through a salt bridge, which maintains the electrical neutrality by preventing the accumulation of the charges by maintaining a free path for the migration of ions.
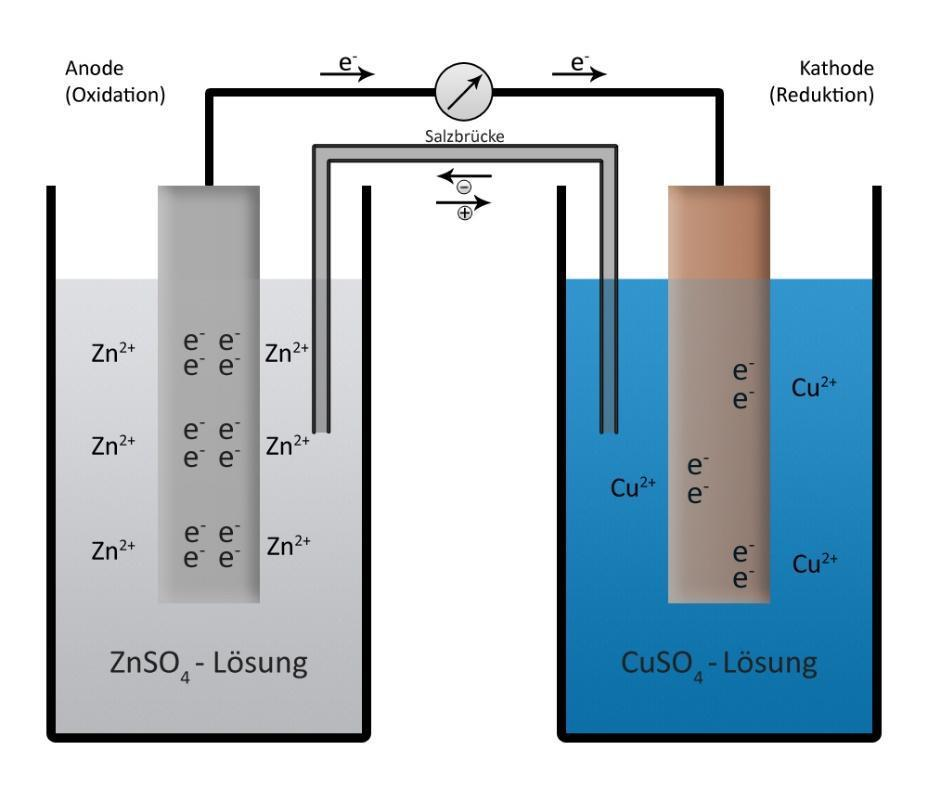
Using half-cell potentials, the electrochemical series and the relative oxidising power or reducing power of a half-reaction. We know that the Daniel cell converts chemical energy into electrical energy. Lower standard reduction potential is of anode and cathode has higher standard reduction potential. Emf of Daniel cell is,
\[{E_{cathode}} - {E_{anode}} = emf\]
So emf of cell has to be greater than zero for working of cell that is Emf >0
Option A: since anode as lower standard potential. So it can’t be greater than zero. B can’t act as anode. This option is wrong.
Option B: As cathode as higher standard potential but the value of A is less than zero. A can’t act as a cathode. This option is wrong.
Option C: here cathode has higher standard potential and the value of B also greater than zero. B can act as a cathode. This option is correct.
Option D: This solution can be predicted through emf. This option is also wrong.
So, the correct answer is “Option c”.
Note:
In electrolytic cells, positively electrode is anode and negatively charged electrode is cathode. In galvanic cells, it is opposite. Positively charged electrode may be a cathode and a negatively charged electrode is anode.
Complete answer:
We have to remember that a Daniel cell may be a galvanic cell which converts the chemical energy into the electrical energy. It consists of two half cells. One half cell is a beaker containing a strip of metallic zinc dipped in 1M aqueous zinc sulfate solution. The second half cell consists of a beaker having a metallic strip of copper immersed in 1M aqueous copper sulfate solution.
The construction of the Daniel cell includes a copper container which is crammed with the diluted sulphuric acid immersed within the copper container. The two different metals are connected to each other through a salt bridge, which maintains the electrical neutrality by preventing the accumulation of the charges by maintaining a free path for the migration of ions.

Using half-cell potentials, the electrochemical series and the relative oxidising power or reducing power of a half-reaction. We know that the Daniel cell converts chemical energy into electrical energy. Lower standard reduction potential is of anode and cathode has higher standard reduction potential. Emf of Daniel cell is,
\[{E_{cathode}} - {E_{anode}} = emf\]
So emf of cell has to be greater than zero for working of cell that is Emf >0
Option A: since anode as lower standard potential. So it can’t be greater than zero. B can’t act as anode. This option is wrong.
Option B: As cathode as higher standard potential but the value of A is less than zero. A can’t act as a cathode. This option is wrong.
Option C: here cathode has higher standard potential and the value of B also greater than zero. B can act as a cathode. This option is correct.
Option D: This solution can be predicted through emf. This option is also wrong.
So, the correct answer is “Option c”.
Note:
In electrolytic cells, positively electrode is anode and negatively charged electrode is cathode. In galvanic cells, it is opposite. Positively charged electrode may be a cathode and a negatively charged electrode is anode.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




