
In a compound
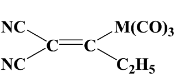
The numbers of sigma and pi bonds respectively are:-
(a) 19, 11
(b) 19, 5
(c) 13, 11
(d) 7, 3

Answer
537.9k+ views
Hint:Approach this question by making an expanded structure of the given compound so that we can clearly see each and every bond by which atoms are connected. If there is only one bond between 2 atoms then it is a sigma bond and if there is more than 1 bond between 2 atoms even then there is one sigma bond and rest are pi bonds.
Complete step-by-step answer:Before solving anything, let’s discuss the chemistry of sigma and pi bonds.
Sigma and pi bonds are among the types of covalent bonds that can be distinguished by the type of overlap between 2 atomic orbitals. Covalent bond is formed by overlapping of orbitals (or we may say by sharing electron pairs).
-Sigma Bond$(\sigma )$ : These are the strongest type of covalent bond as they are formed by head-to-head overlapping of atomic orbitals. The electrons participating in sigma bonds are known as sigma electrons. These bonds can exist independently and they play a role in determining the shape of the molecule.
-Pi Bond$(\pi )$: these bonds are relatively weaker bonds and are formed by side-by-side overlapping of atomic orbitals. The electrons participating in pi bond formation are known as pi electrons. These bonds can’t exist independently without sigma bonds. Also they play no role in determining the shape of molecules.
Generally, if (A) single bond: 1 $\sigma $ bond
(B) Double bond: 1 $\sigma $ bond, 1 $\pi $ bond
(C) Triple bond: 1 $\sigma $ bond, 2 $\pi $ bonds and so on.
Now let’s draw the expanded structure of the given compound so as to clearly see the number of bonds between each and every atom.
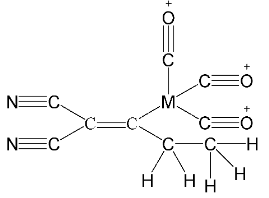
As we can see that, each atom is connected by at least one single bond which will be counted as $\sigma $ bonds and the rest are $\pi $ bonds.
So, the total number of sigma bonds are: 19 and the total number of pi bonds are: 11
Therefore, the correct option is: (a) 19, 11
Note:Here in case of CO, always include coordinate covalent bond in pi bond while counting them.( coordinate covalent bond is formed when only one atom contributes in sharing of electron pair and other atom accepts the electron pair to complete its octet.
-If we have to only calculate sigma bonds then we can also use an alternative method without making the complete structure of the compound (only for carbon containing compounds) by the given formula:-
No. of sigma bonds= [(No. of carbon atom)-1] + [Total no. of other atoms] in the given compound.
For the above compound:-
Total no. of C atom = 9 Total no. of other atoms =11
No. of sigma bonds = (9-1) +11 = 19
Complete step-by-step answer:Before solving anything, let’s discuss the chemistry of sigma and pi bonds.
Sigma and pi bonds are among the types of covalent bonds that can be distinguished by the type of overlap between 2 atomic orbitals. Covalent bond is formed by overlapping of orbitals (or we may say by sharing electron pairs).
-Sigma Bond$(\sigma )$ : These are the strongest type of covalent bond as they are formed by head-to-head overlapping of atomic orbitals. The electrons participating in sigma bonds are known as sigma electrons. These bonds can exist independently and they play a role in determining the shape of the molecule.
-Pi Bond$(\pi )$: these bonds are relatively weaker bonds and are formed by side-by-side overlapping of atomic orbitals. The electrons participating in pi bond formation are known as pi electrons. These bonds can’t exist independently without sigma bonds. Also they play no role in determining the shape of molecules.
Generally, if (A) single bond: 1 $\sigma $ bond
(B) Double bond: 1 $\sigma $ bond, 1 $\pi $ bond
(C) Triple bond: 1 $\sigma $ bond, 2 $\pi $ bonds and so on.
Now let’s draw the expanded structure of the given compound so as to clearly see the number of bonds between each and every atom.

As we can see that, each atom is connected by at least one single bond which will be counted as $\sigma $ bonds and the rest are $\pi $ bonds.
So, the total number of sigma bonds are: 19 and the total number of pi bonds are: 11
Therefore, the correct option is: (a) 19, 11
Note:Here in case of CO, always include coordinate covalent bond in pi bond while counting them.( coordinate covalent bond is formed when only one atom contributes in sharing of electron pair and other atom accepts the electron pair to complete its octet.
-If we have to only calculate sigma bonds then we can also use an alternative method without making the complete structure of the compound (only for carbon containing compounds) by the given formula:-
No. of sigma bonds= [(No. of carbon atom)-1] + [Total no. of other atoms] in the given compound.
For the above compound:-
Total no. of C atom = 9 Total no. of other atoms =11
No. of sigma bonds = (9-1) +11 = 19
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




