
In a cell, electrons move from:
a) positive electrode to negative electrode
b) negative electrode to positive electrode
c) both a and b
d) electrons do not move and only negative charges move from one place to another place.
Answer
590.7k+ views
Hint: The electrons are negatively charged particles. By nature the charges having the same nature repel each other and the charges of opposite nature attract each other. In a cell, a battery consists of two electrodes i.e. positive and negative electrodes. From this information we can easily come to an answer.
Complete step-by-step answer:
To begin with let us draw a diagram to visualize the electrons flow in a wire connected to a battery.
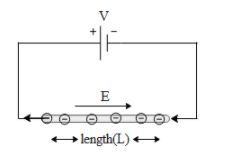
In the above figure a metal rod is connected to a battery of emf V. The length of the rod is L. Hence we can say that there exists a electric field across the length of the rod which is given by,
$E=\dfrac{V}{L}$ and it is directed from the positive terminal of the battery to the negative terminal of the battery. If a negative charge is placed in the field it will experience a force in the direction opposite to the applied electric field. Since electrons are negatively charged, they will move from the negative electrode of the battery to the positive electrode of the battery.
So, the correct answer is “Option b”.
Note: It is to be noted that the cell uses its chemical energy to deliver the electrons from the positive to the negative electrode of the battery. This is how we get a constant current supply when connected to a cell having some emf. So inside the cell the electrons the electrons are forced from the positive to the negative electrode of the cell.
Complete step-by-step answer:
To begin with let us draw a diagram to visualize the electrons flow in a wire connected to a battery.

In the above figure a metal rod is connected to a battery of emf V. The length of the rod is L. Hence we can say that there exists a electric field across the length of the rod which is given by,
$E=\dfrac{V}{L}$ and it is directed from the positive terminal of the battery to the negative terminal of the battery. If a negative charge is placed in the field it will experience a force in the direction opposite to the applied electric field. Since electrons are negatively charged, they will move from the negative electrode of the battery to the positive electrode of the battery.
So, the correct answer is “Option b”.
Note: It is to be noted that the cell uses its chemical energy to deliver the electrons from the positive to the negative electrode of the battery. This is how we get a constant current supply when connected to a cell having some emf. So inside the cell the electrons the electrons are forced from the positive to the negative electrode of the cell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




