
In a Cannizzaro reaction, the intermediate that will be the best hydride donor is:
A.
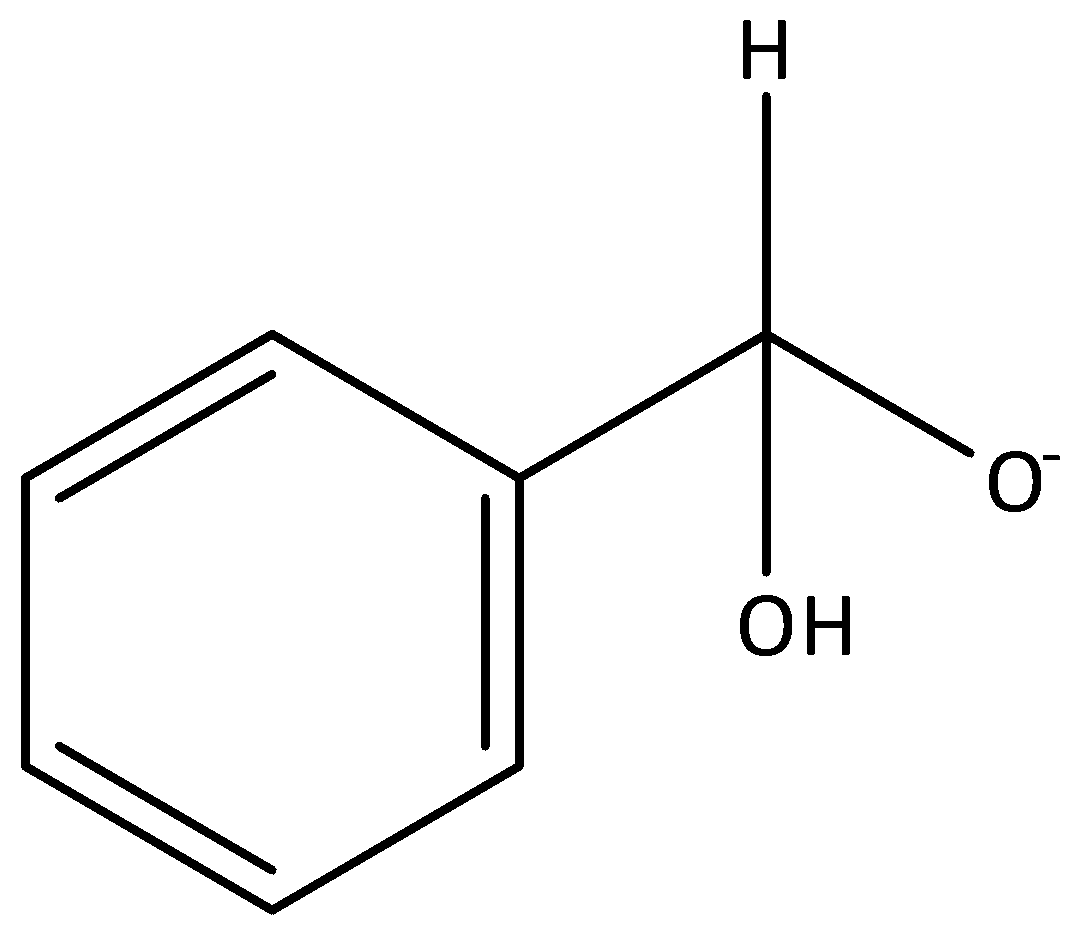
B.

C.
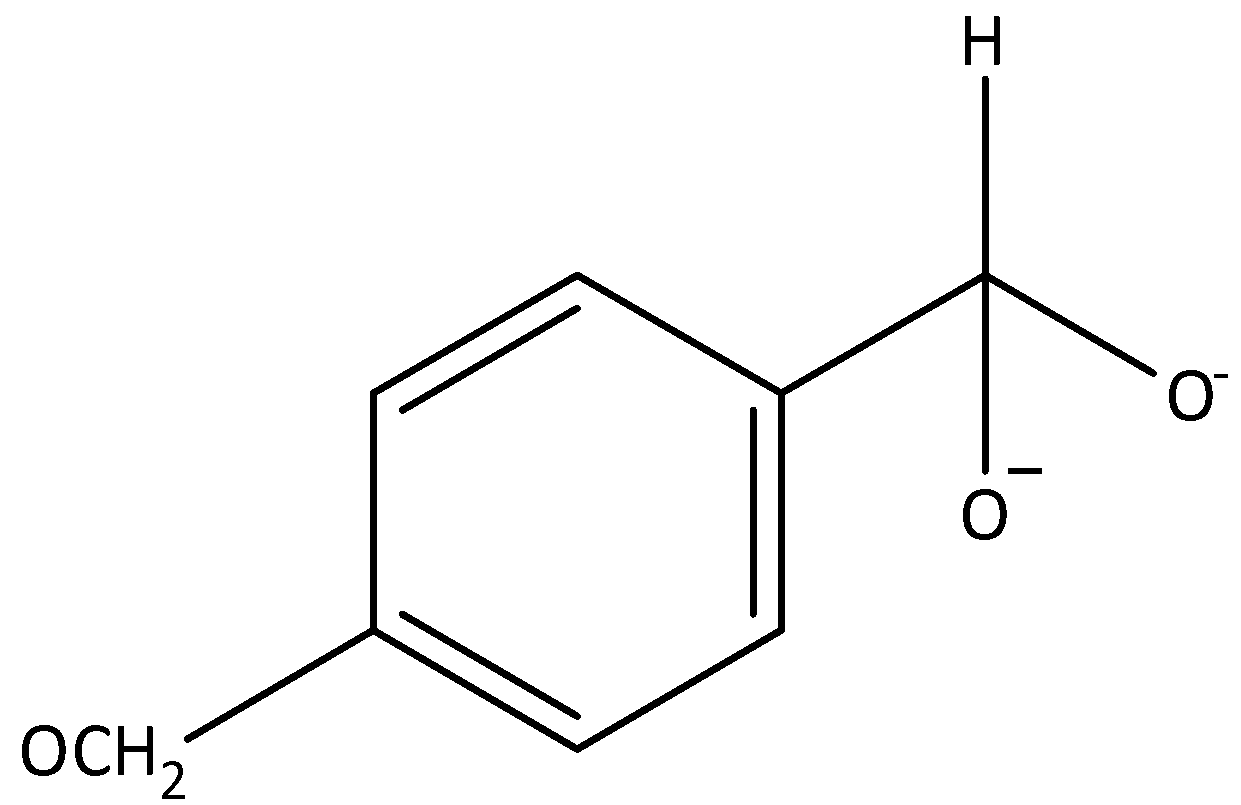
D.
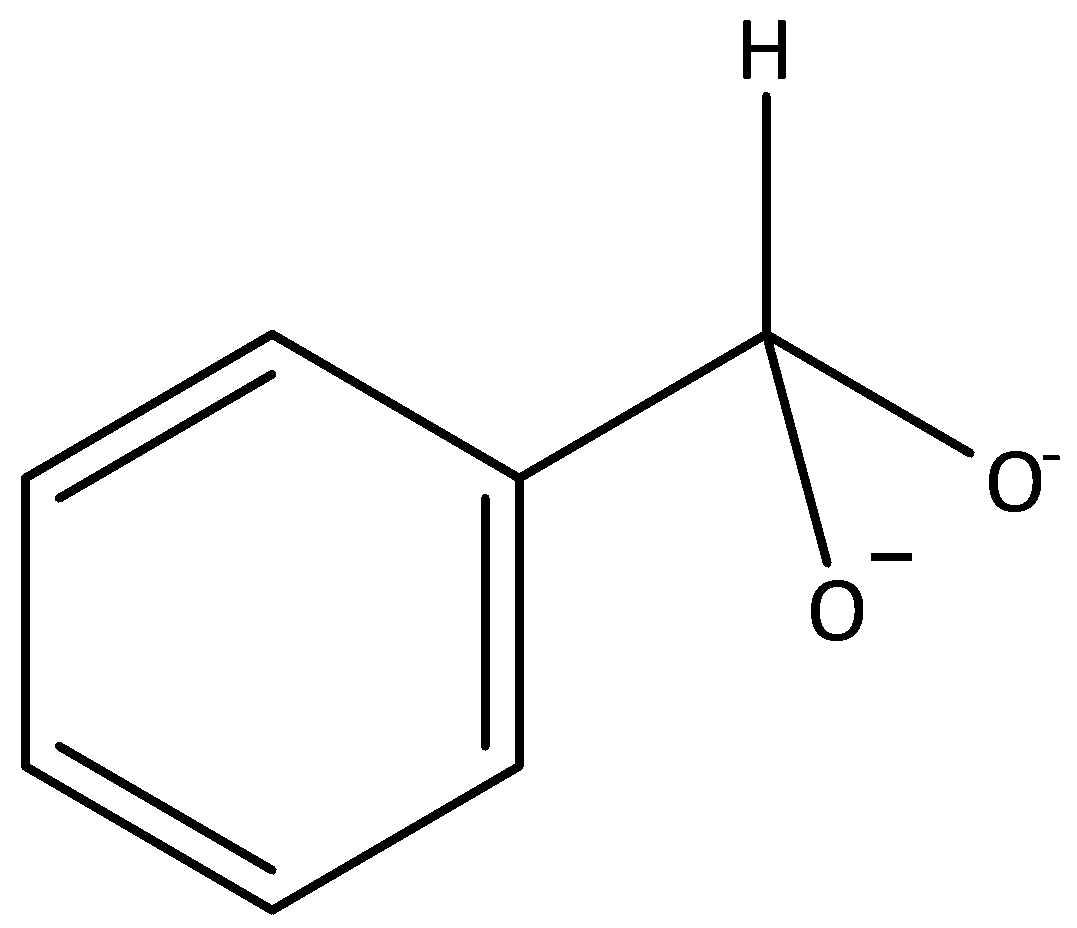




Answer
583.2k+ views
Hint:We know that Cannizzaro reaction is a chemical reaction, which includes the base-induced disproportionation of two molecules of an aldehyde to form a carboxylic acid and a primary alcohol.
Complete step by step answer:
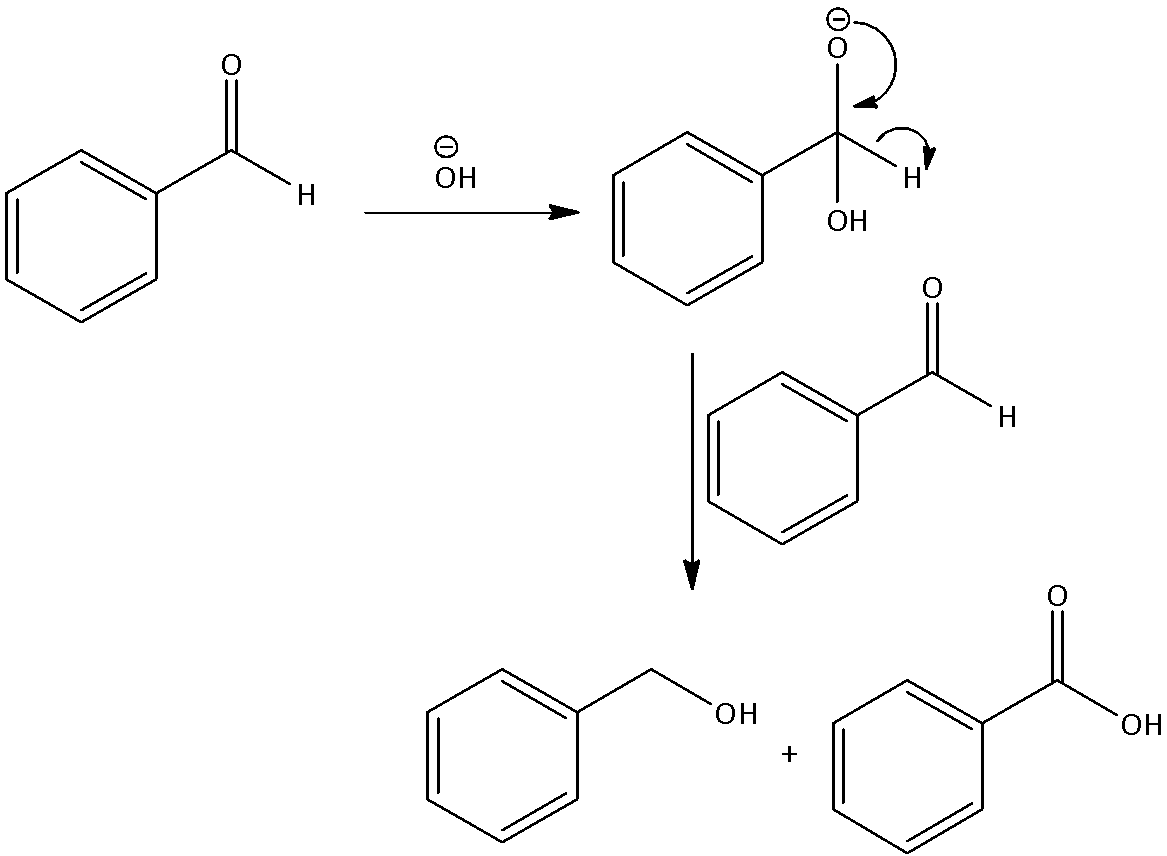
The mechanism of Cannizzaro reaction describes the method to produce one molecule of alcohol and one molecule of carboxylic acid. The reaction is two molecules of the given aldehyde.
The reaction occurs by a nucleophilic acyl substitution on an aldehyde where the leaving group attacks another aldehyde. A tetrahedral intermediate is formed from the attack of hydroxide on a carbonyl. This tetrahedral intermediate breaks down. Reforming the carbonyl and transferring a hydride that attacks another colony. An exchange of protons takes place by acid and alkoxide ions. When we introduce a base of high concentration, the aldehyde produces an anion that contains a charge of 2. From this, an ion of hydride is transferred to a second molecule of the aldehyde, producing carboxylate and alkoxide ions. The alkoxide ion also abstracts a proton from the solvent for the reaction.
We can write the mechanism as,
In a Cannizzaro reaction, the intermediate that would be the best hydride donor is indicated by option A. It is formed when hydroxide ion attacks a molecule of benzaldehyde. The intermediates that are given in options B, C and D cannot be formed in this reaction.
Therefore, the option (A) is correct.
Note: We must know that the variation of the reaction enhances the yield of the desired product. Both the aldehydes used are completely converted to products and wastage of the valuable reactant chemicals is avoided. The atom economy of the process is very low. Since both oxidation and reduction takes place in the transfer of hydride, the reaction is called a redox process.
Complete step by step answer:
The mechanism of Cannizzaro reaction describes the method to produce one molecule of alcohol and one molecule of carboxylic acid. The reaction is two molecules of the given aldehyde.
The reaction occurs by a nucleophilic acyl substitution on an aldehyde where the leaving group attacks another aldehyde. A tetrahedral intermediate is formed from the attack of hydroxide on a carbonyl. This tetrahedral intermediate breaks down. Reforming the carbonyl and transferring a hydride that attacks another colony. An exchange of protons takes place by acid and alkoxide ions. When we introduce a base of high concentration, the aldehyde produces an anion that contains a charge of 2. From this, an ion of hydride is transferred to a second molecule of the aldehyde, producing carboxylate and alkoxide ions. The alkoxide ion also abstracts a proton from the solvent for the reaction.
We can write the mechanism as,

In a Cannizzaro reaction, the intermediate that would be the best hydride donor is indicated by option A. It is formed when hydroxide ion attacks a molecule of benzaldehyde. The intermediates that are given in options B, C and D cannot be formed in this reaction.
Therefore, the option (A) is correct.
Note: We must know that the variation of the reaction enhances the yield of the desired product. Both the aldehydes used are completely converted to products and wastage of the valuable reactant chemicals is avoided. The atom economy of the process is very low. Since both oxidation and reduction takes place in the transfer of hydride, the reaction is called a redox process.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




