
In 3-methyl-2-cyclohexenone which hydrogen cannot undergo deuterium exchange when it reacts with $ C{H_3}{{\text{O}}^\Theta }/C{H_3}OD $ ?
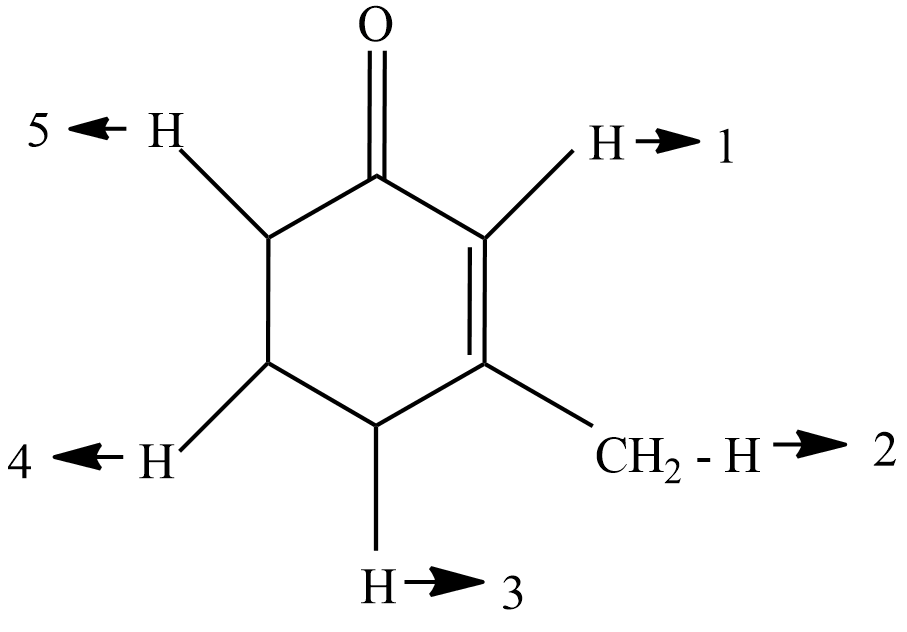
(A) $ {{\text{H}}_1}\;,\;{{\text{H}}_4} $
(B) $ \;{{\text{H}}_4} $
(C) $ {{\text{H}}_3}\;,\;{{\text{H}}_2} $
(D) $ {{\text{H}}_5}\;,\;{{\text{H}}_3} $

Answer
512.7k+ views
Hint : This question talks abouts exchange of isotopes of Hydrogen. For this kind of question, we have to know about the Kinetic Isotopic effect in Tautomerism which says If we take a heavier isotope of Hydrogen then all the $ {\text{\alpha }} $ Hydrogen and the hydrogen which are in conjugation with the carbonyl group will be replaced by the heavier isotope of Hydrogen. So, in this question we will identify the $ {\text{\alpha }} $ Hydrogens and replace them with the heavier Isotope.
Complete Step By Step Answer:
Now, if we see in the diagram $ {{\text{H}}_1} $ and $ {{\text{H}}_5} $ are the alpha hydrogen because they are directly connected to the carbonyl group, so they will react with $ C{H_3}{{\text{O}}^\Theta }/C{H_3}OD $ they will get replaced. now $ {{\text{H}}_2} $ and $ {{\text{H}}_3} $ hydrogen are in conjugated hydrogen they will also get replaced when reacted with ch3 now we are left the $ {{\text{H}}_4} $ hydrogen if we see the diagram then it is clear that $ {{\text{H}}_4} $ hydrogen is neither a alpha hydrogen nor a conjugated hydrogen so it will not react with the $ C{H_3}{{\text{O}}^\Theta }/C{H_3}OD $ and will not be replaced.
The carbon atoms directly attached to the functional group are known as $ {\text{\alpha }} $ Carbon, Hydrogen atoms attached to $ {\text{\alpha }} $ carbon atoms are $ {\text{\alpha }} $ Hydrogen. Since they are directly attached to the $ {\text{\alpha }} $ carbon they become more reactive. So carefully observe the diagram to identify them.
The reaction taking place will be
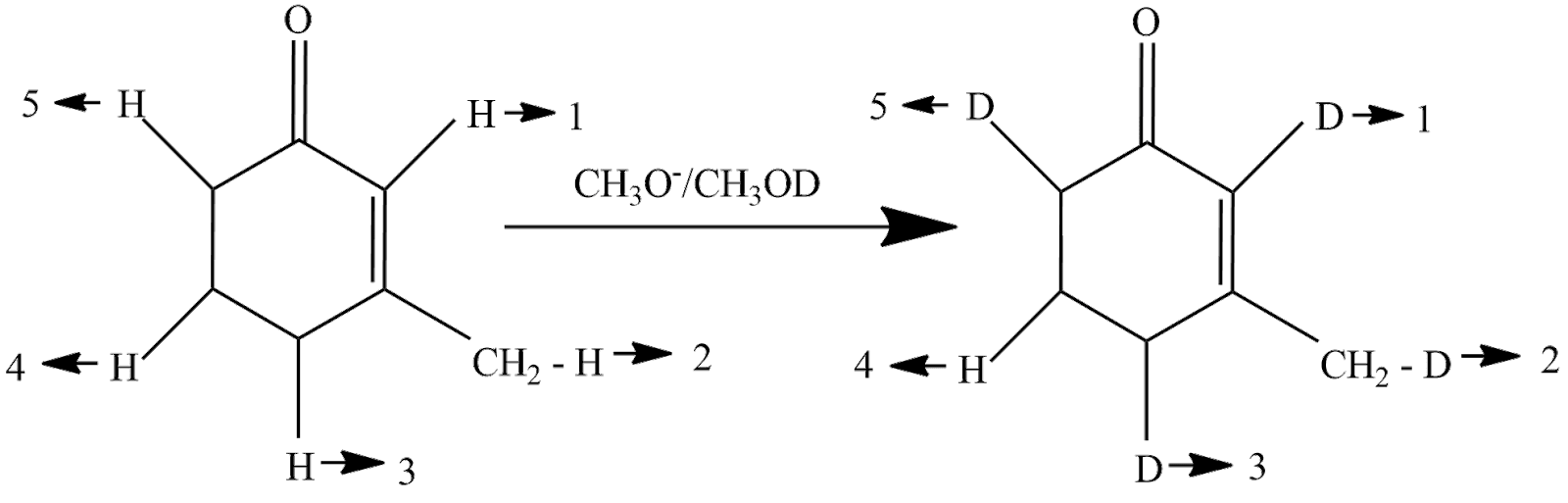
So, the answer is option (C).
Note :
Deuterium is an isotope of hydrogen also known as heavy Hydrogen with a nucleus containing one proton and one neutron so it has twice the mass of a normal hydrogen. When Deuterium is reacted with oxygen it forms $ {D_2}O $ also known as Heavy Water.
Complete Step By Step Answer:
Now, if we see in the diagram $ {{\text{H}}_1} $ and $ {{\text{H}}_5} $ are the alpha hydrogen because they are directly connected to the carbonyl group, so they will react with $ C{H_3}{{\text{O}}^\Theta }/C{H_3}OD $ they will get replaced. now $ {{\text{H}}_2} $ and $ {{\text{H}}_3} $ hydrogen are in conjugated hydrogen they will also get replaced when reacted with ch3 now we are left the $ {{\text{H}}_4} $ hydrogen if we see the diagram then it is clear that $ {{\text{H}}_4} $ hydrogen is neither a alpha hydrogen nor a conjugated hydrogen so it will not react with the $ C{H_3}{{\text{O}}^\Theta }/C{H_3}OD $ and will not be replaced.
The carbon atoms directly attached to the functional group are known as $ {\text{\alpha }} $ Carbon, Hydrogen atoms attached to $ {\text{\alpha }} $ carbon atoms are $ {\text{\alpha }} $ Hydrogen. Since they are directly attached to the $ {\text{\alpha }} $ carbon they become more reactive. So carefully observe the diagram to identify them.
The reaction taking place will be

So, the answer is option (C).
Note :
Deuterium is an isotope of hydrogen also known as heavy Hydrogen with a nucleus containing one proton and one neutron so it has twice the mass of a normal hydrogen. When Deuterium is reacted with oxygen it forms $ {D_2}O $ also known as Heavy Water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




