
If the quantum numbers for the \[{5^{th}}\] electron in the carbon atom are $2,1, - 1, + \dfrac{1}{2}$ , then for the ${6^{th}}$ electron, these values would be:
A.$1,1,0, - \dfrac{1}{2}$
B.$2,0,1, + \dfrac{1}{2}$
C.$2,1,1, - \dfrac{1}{2}$
D.$2,1,0, + \dfrac{1}{2}$
Answer
576.6k+ views
Hint: We know that the value of n for p orbital is already given, we know that l=$n - 1$ and total value of m=$2l + 1$ where m has values ranging from –l to +l. The value of spin quantum number is $ + \dfrac{1}{2}$ for clockwise rotation and $ - \dfrac{1}{2}$ for anticlockwise rotation of electrons. Write the electronic configuration of carbon atoms and find these values.
Complete step by step answer:
Given, the quantum numbers for the \[{5^{th}}\] electron in the carbon atom are $2,1, - 1, + \dfrac{1}{2}$ and we have to find the values of all quantum numbers for ${6^{th}}$ electron of carbon atom.
We know that carbon has atomic number $6$ and the electronic configuration of electrons is $1{s^2},2{s^2}2{p^2}$
The representation of electrons in the orbital is given as-
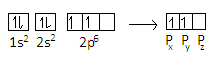
We can see from the representation that the \[{5^{th}}\] electron in the carbon atom is in $2{p_x}$ orbital and ${6^{th}}$ electron of carbon atom is in$2{p_y}$.For fifth electron, the principal quantum number n=$2$ , azimuthal quantum number l=$1$ , magnetic quantum number m=$ - 1$ and spin quantum number s=$ + \dfrac{1}{2}$ For the sixth electron, n=$2$and l=$1$ as the principal quantum and azimuthal quantum number will remain the same as the electron is in p=orbital.We know that total values of m=$2l + 1$ and can have values from –l to +l.On putting value of l in the formula, we get-
$ \Rightarrow $ m=$2 + 1 = 3$
So there will be three values of m ranging from $ - 1$to $ + 1$but since the fifth electron already has one value of m=$1$ so either m=$0{\text{ or - 1}}$ Since the direction of the electron is same as that of the fifth electron then the value of s will also be same .i.e. $ + \dfrac{1}{2}$ So the values of the sixth electron’s quantum numbers are-$2,1,{\text{ - 1}}, + \dfrac{1}{2}$ .
Hence the correct answer is option D.
Note:
Quantum numbers are a set of numbers that tell the position and energy of an electron in an atom. Each quantum number tells different points about the electron-
-The principal quantum number ‘n’ tells about the number of the principal shell in which the electron is present.
-Azimuthal quantum number gives the shape of an orbital and is equal to the total number angular nodes of an orbital.
-Magnetic quantum number tells about the orientation and the total number of orbitals of a subshell.
-Spin quantum number gives the direction of the spin of the electron in the orbital.
Complete step by step answer:
Given, the quantum numbers for the \[{5^{th}}\] electron in the carbon atom are $2,1, - 1, + \dfrac{1}{2}$ and we have to find the values of all quantum numbers for ${6^{th}}$ electron of carbon atom.
We know that carbon has atomic number $6$ and the electronic configuration of electrons is $1{s^2},2{s^2}2{p^2}$
The representation of electrons in the orbital is given as-
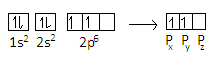
We can see from the representation that the \[{5^{th}}\] electron in the carbon atom is in $2{p_x}$ orbital and ${6^{th}}$ electron of carbon atom is in$2{p_y}$.For fifth electron, the principal quantum number n=$2$ , azimuthal quantum number l=$1$ , magnetic quantum number m=$ - 1$ and spin quantum number s=$ + \dfrac{1}{2}$ For the sixth electron, n=$2$and l=$1$ as the principal quantum and azimuthal quantum number will remain the same as the electron is in p=orbital.We know that total values of m=$2l + 1$ and can have values from –l to +l.On putting value of l in the formula, we get-
$ \Rightarrow $ m=$2 + 1 = 3$
So there will be three values of m ranging from $ - 1$to $ + 1$but since the fifth electron already has one value of m=$1$ so either m=$0{\text{ or - 1}}$ Since the direction of the electron is same as that of the fifth electron then the value of s will also be same .i.e. $ + \dfrac{1}{2}$ So the values of the sixth electron’s quantum numbers are-$2,1,{\text{ - 1}}, + \dfrac{1}{2}$ .
Hence the correct answer is option D.
Note:
Quantum numbers are a set of numbers that tell the position and energy of an electron in an atom. Each quantum number tells different points about the electron-
-The principal quantum number ‘n’ tells about the number of the principal shell in which the electron is present.
-Azimuthal quantum number gives the shape of an orbital and is equal to the total number angular nodes of an orbital.
-Magnetic quantum number tells about the orientation and the total number of orbitals of a subshell.
-Spin quantum number gives the direction of the spin of the electron in the orbital.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




