
If the number of solute particles in chamber A is increased, then which of the following statements is correct?
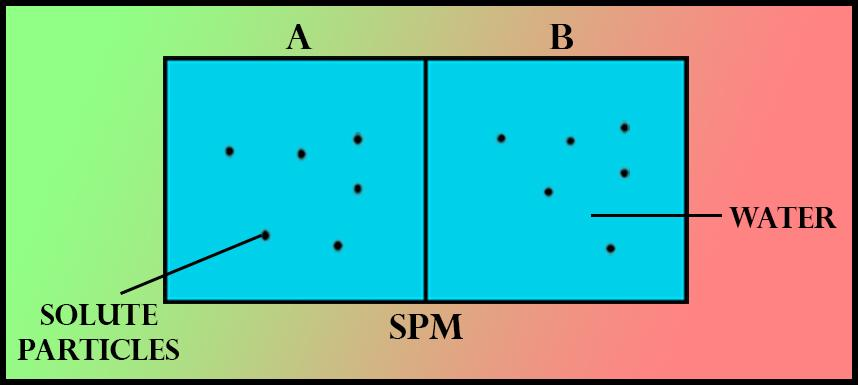
(a)The rate of${ H }_{ 2 }{ O }$ movement through semipermeable membranes(SPM) is decreased.
(b)Water molecules in chamber 'A' attain more free energy.
(c)Water molecules in chamber 'B' show more or increased rate of movement.
(d)The water potential of chamber 'B' is increased.

Answer
584.1k+ views
Hint: In osmosis, molecules of a solvent tend to pass through a semipermeable membrane from a less concentrated solution into a more concentrated one. If a solution container is separated by a semipermeable membrane and concentration is increased on one side, the solvent from the other side will rush to balance the concentration.
Complete answer:
If the number of solute particles in chamber ‘A’ is increased, then water molecules in chamber 'B' show more or increased rate of movement and they will start moving in solution ‘A’. It is clear from the given diagram that the experiment conducted is related to the process of osmosis. Here, chamber ‘A’ and chamber ‘B’ are separated by a semipermeable membrane. Both the chambers ‘A’ and ‘B’ contain water as a solvent and solute particles. If the number of solute practices in chamber ‘A’ is increased, then it will increase the concentration of the solution in chamber ‘A’. This means that the concentration of the solution in Chamber ‘A’ has become higher than the solution in Chamber ‘B’. Since the solvent is water so according to the osmosis phenomenon the water molecules in chamber 'B' show more or increased rate of movement and they will move towards a solution ‘A’ to maintain the concentration equal in both sides of the semipermeable membrane.
So, the correct answer is, ’Water molecules in chamber 'B' show more or increased rate of movement’.
Note: -The process of osmosis is very much important in biology and was first thoroughly studied in 1877 by a German plant physiologist, Wilhelm Pfeffer.
-One of the best example processes in the human body is, when red blood cells, with a high concentration of protein and salt, are placed in a lower concentration fluid like water, the water will rush into the red blood cells.
-With the help of Osmosis, plants take water and minerals from the roots.
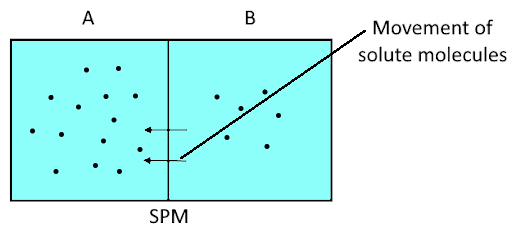
Complete answer:
If the number of solute particles in chamber ‘A’ is increased, then water molecules in chamber 'B' show more or increased rate of movement and they will start moving in solution ‘A’. It is clear from the given diagram that the experiment conducted is related to the process of osmosis. Here, chamber ‘A’ and chamber ‘B’ are separated by a semipermeable membrane. Both the chambers ‘A’ and ‘B’ contain water as a solvent and solute particles. If the number of solute practices in chamber ‘A’ is increased, then it will increase the concentration of the solution in chamber ‘A’. This means that the concentration of the solution in Chamber ‘A’ has become higher than the solution in Chamber ‘B’. Since the solvent is water so according to the osmosis phenomenon the water molecules in chamber 'B' show more or increased rate of movement and they will move towards a solution ‘A’ to maintain the concentration equal in both sides of the semipermeable membrane.
So, the correct answer is, ’Water molecules in chamber 'B' show more or increased rate of movement’.
Note: -The process of osmosis is very much important in biology and was first thoroughly studied in 1877 by a German plant physiologist, Wilhelm Pfeffer.
-One of the best example processes in the human body is, when red blood cells, with a high concentration of protein and salt, are placed in a lower concentration fluid like water, the water will rush into the red blood cells.
-With the help of Osmosis, plants take water and minerals from the roots.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




