
If “n” number of \[{{H}_{3}}P{{O}_{4}}\] molecules are polymerized to produce chain molecule and ring molecule separately, then the number of \[P-O-P\] linkage forms is, respectively.
(A) n and (n-1)
(B) (n-1) and (n-1)
(C) (n-1) and n
(D) n and n
Answer
585.3k+ views
Hint: Polymerization is a method or a process in which monomer molecules react together through chemical reaction to form polymer chains or three-dimensional network structure.
Complete step by step answer:
\[{{H}_{3}}P{{O}_{4}}\] means phosphoric acid is a monomer that undergoes polymerization forms two types of products.
One type of product is ring structure based molecule.
Second type is a chain type structure based molecule.
In case of a ring structure, the number of \[P-O-P\]linkages will be one less (n-1) than the number of phosphorus atoms (\[P\]) because of the fact that each phosphorus atom is shared by two rings in the structure.
Though, in case of a chain structure there is no sharing and the number of \[P-O-P\] linkages is equal to the number of phosphorus atoms (n).
So, the “n-1” \[P-O-P\] linkages in case of ring structure.
“n”\[P-O-P\]linkages in case of chain structure.
Therefore “n-1” linkages are present in ring structure and “n” linkages are present in chain structure produced by the polymerisation of phosphoric acid.
So, the correct option is A, n and (n-1).
Note: Don’t be confused in the given options A and C.
In chain structure “n”\[P-O-P\] linkages and in ring structure “(n-1)” \[P-O-P\] linkages.
Don’t be confused with ring structure and chain structure.
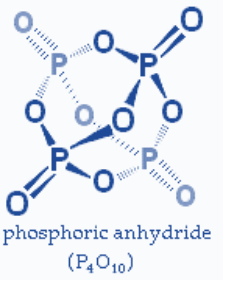
Ring structure
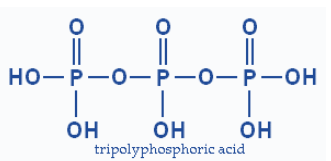
Chain structure
Complete step by step answer:
\[{{H}_{3}}P{{O}_{4}}\] means phosphoric acid is a monomer that undergoes polymerization forms two types of products.
One type of product is ring structure based molecule.
Second type is a chain type structure based molecule.
In case of a ring structure, the number of \[P-O-P\]linkages will be one less (n-1) than the number of phosphorus atoms (\[P\]) because of the fact that each phosphorus atom is shared by two rings in the structure.
Though, in case of a chain structure there is no sharing and the number of \[P-O-P\] linkages is equal to the number of phosphorus atoms (n).
So, the “n-1” \[P-O-P\] linkages in case of ring structure.
“n”\[P-O-P\]linkages in case of chain structure.
Therefore “n-1” linkages are present in ring structure and “n” linkages are present in chain structure produced by the polymerisation of phosphoric acid.
So, the correct option is A, n and (n-1).
Note: Don’t be confused in the given options A and C.
In chain structure “n”\[P-O-P\] linkages and in ring structure “(n-1)” \[P-O-P\] linkages.
Don’t be confused with ring structure and chain structure.

Ring structure

Chain structure
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




