
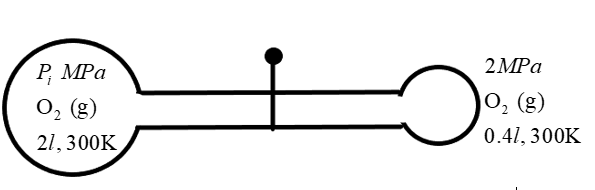
If in the below diagram after opening the valve, final pressure is \[\dfrac{7}{6}MPa\], then find the initial pressure \[{{P}_{1}}\text{ }(in\text{ }MPa)\].

Answer
579.3k+ views
Hint: We are given two containers connected by a pipe which has unequal pressure and volume conditions initially. The thermodynamic variables are kept steady by separating them with a valve, which is opened later. We can use the gas law to solve the unknown variable.
Complete step by step answer:
We are given a condition in which unequal states of thermodynamic conditions mix with each other resulting in another state. Two bulb-like containers of volume \[{{\text{V}}_{1\,}}\text{ and }{{\text{V}}_{2}}\] contains gaseous oxygen at pressure \[{{P}_{1}}\text{ and }{{P}_{2}}\]respectively at a temperature 300K. We know that the gaseous oxygen tends to have a different number of molecules at different thermodynamic conditions. So, initially the number of molecules in the containers are given as \[{{n}_{1}}\text{ and }{{\text{n}}_{2}}\] respectively.
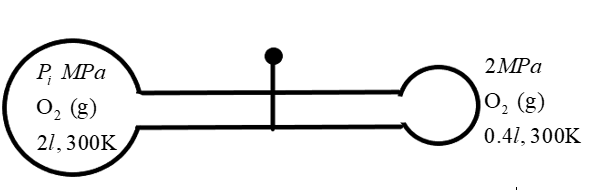
Now, let us consider the situation when the valve is opened. We know for sure that the oxygen molecules tend to move across the pipe due to the pressure difference at the two ends. So, what we have to calculate is the resulting conditions which will be observed as a result of this opening.
For this, we can apply the gas law. The gas law is given as –
\[PV=nRT\]
Where, P is the pressure in the container,
V is the volume of the container,
n is the number of molecules in the container,
R is the gas constant, and,
T is the temperature in absolute scale.
Let us consider this gas law equation for the two containers in the initial condition.
For first container,
\[\begin{align}
& {{P}_{1}}{{V}_{1}}={{n}_{1}}RT \\
& \therefore\ {{n}_{1}}=\dfrac{{{P}_{1}}{{V}_{1}}}{RT}\text{ -(1)} \\
\end{align}\]
For the second container,
\[\begin{align}
& {{P}_{2}}{{V}_{2}}={{n}_{2}}RT \\
& \therefore\ {{n}_{2}}=\dfrac{{{P}_{2}}{{V}_{2}}}{RT}\text{ --(2)} \\
\end{align}\]
From, (1) and (2), we get the number of molecules present in each container initially.
Now, let us consider the final condition where the valve is opened. In this case, we have to remember that the total number of molecules in the system remains constant, i.e., n is the sum of the number of molecules in both containers.
Applying the gas law to the final condition,
\[\begin{align}
& {{P}_{f}}{{V}_{f}}=nRT \\
& \therefore \ n=\dfrac{{{P}_{f}}{{V}_{f}}}{RT} \\
\end{align}\]
Now,
\[\begin{align}
& n={{n}_{1}}+{{n}_{2}} \\
& \Rightarrow \text{ }\dfrac{{{P}_{f}}{{V}_{f}}}{RT}=\dfrac{{{P}_{1}}{{V}_{1}}}{RT}+\dfrac{{{P}_{2}}{{V}_{2}}}{RT} \\
\end{align}\]
We can find the required pressure which is \[{{P}_{1}}\] by substituting given values in the above equation as –
\[\begin{align}
& {{P}_{f}}=\dfrac{7}{6}MPa \\
& {{P}_{2}}=2MPa \\
& {{V}_{1}}=2l \\
& {{V}_{2}}=0.4l \\
& {{V}_{f}}=2.4l \\
& \\
\end{align}\]
\[\begin{align}
& {{P}_{f}}{{V}_{f}}={{P}_{1}}{{V}_{1}}+{{P}_{2}}{{V}_{2}} \\
& \Rightarrow \text{ }\dfrac{7}{6}(2.4)={{P}_{1}}(2)+2(0.4) \\
& \Rightarrow \text{ }2.8=2{{P}_{1}}+0.8 \\
& \Rightarrow \text{ }{{P}_{1}}=\dfrac{2.8-0.8}{2} \\
& \therefore\text{ }{{P}_{1}}=1MPa \\
\end{align}\]
The required pressure condition is 1 MPa initially in the first container.
Note:
We use the ideal gas condition for solving the thermodynamic variables when the temperature and pressure conditions are moderate. Most of the gases in normal temperatures and pressure conditions do not deviate much from the ideal gas conditions.
Complete step by step answer:
We are given a condition in which unequal states of thermodynamic conditions mix with each other resulting in another state. Two bulb-like containers of volume \[{{\text{V}}_{1\,}}\text{ and }{{\text{V}}_{2}}\] contains gaseous oxygen at pressure \[{{P}_{1}}\text{ and }{{P}_{2}}\]respectively at a temperature 300K. We know that the gaseous oxygen tends to have a different number of molecules at different thermodynamic conditions. So, initially the number of molecules in the containers are given as \[{{n}_{1}}\text{ and }{{\text{n}}_{2}}\] respectively.

Now, let us consider the situation when the valve is opened. We know for sure that the oxygen molecules tend to move across the pipe due to the pressure difference at the two ends. So, what we have to calculate is the resulting conditions which will be observed as a result of this opening.
For this, we can apply the gas law. The gas law is given as –
\[PV=nRT\]
Where, P is the pressure in the container,
V is the volume of the container,
n is the number of molecules in the container,
R is the gas constant, and,
T is the temperature in absolute scale.
Let us consider this gas law equation for the two containers in the initial condition.
For first container,
\[\begin{align}
& {{P}_{1}}{{V}_{1}}={{n}_{1}}RT \\
& \therefore\ {{n}_{1}}=\dfrac{{{P}_{1}}{{V}_{1}}}{RT}\text{ -(1)} \\
\end{align}\]
For the second container,
\[\begin{align}
& {{P}_{2}}{{V}_{2}}={{n}_{2}}RT \\
& \therefore\ {{n}_{2}}=\dfrac{{{P}_{2}}{{V}_{2}}}{RT}\text{ --(2)} \\
\end{align}\]
From, (1) and (2), we get the number of molecules present in each container initially.
Now, let us consider the final condition where the valve is opened. In this case, we have to remember that the total number of molecules in the system remains constant, i.e., n is the sum of the number of molecules in both containers.
Applying the gas law to the final condition,
\[\begin{align}
& {{P}_{f}}{{V}_{f}}=nRT \\
& \therefore \ n=\dfrac{{{P}_{f}}{{V}_{f}}}{RT} \\
\end{align}\]
Now,
\[\begin{align}
& n={{n}_{1}}+{{n}_{2}} \\
& \Rightarrow \text{ }\dfrac{{{P}_{f}}{{V}_{f}}}{RT}=\dfrac{{{P}_{1}}{{V}_{1}}}{RT}+\dfrac{{{P}_{2}}{{V}_{2}}}{RT} \\
\end{align}\]
We can find the required pressure which is \[{{P}_{1}}\] by substituting given values in the above equation as –
\[\begin{align}
& {{P}_{f}}=\dfrac{7}{6}MPa \\
& {{P}_{2}}=2MPa \\
& {{V}_{1}}=2l \\
& {{V}_{2}}=0.4l \\
& {{V}_{f}}=2.4l \\
& \\
\end{align}\]
\[\begin{align}
& {{P}_{f}}{{V}_{f}}={{P}_{1}}{{V}_{1}}+{{P}_{2}}{{V}_{2}} \\
& \Rightarrow \text{ }\dfrac{7}{6}(2.4)={{P}_{1}}(2)+2(0.4) \\
& \Rightarrow \text{ }2.8=2{{P}_{1}}+0.8 \\
& \Rightarrow \text{ }{{P}_{1}}=\dfrac{2.8-0.8}{2} \\
& \therefore\text{ }{{P}_{1}}=1MPa \\
\end{align}\]
The required pressure condition is 1 MPa initially in the first container.
Note:
We use the ideal gas condition for solving the thermodynamic variables when the temperature and pressure conditions are moderate. Most of the gases in normal temperatures and pressure conditions do not deviate much from the ideal gas conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




