
If Hund’s rule is not followed, magnetic moment of $F{e^{2 + }},M{n^ + }{\text{ and Cr}}$ all having 24 electrons will be in order:
(A) $F{e^{2 + }},M{n^ + } < Cr$
(B) $F{e^{2 + }} = Cr < M{n^ + }$
(C) $F{e^{2 + }} = M{n^ + } < Cr$
(D) $M{n^ + } = Cr < F{e^{2 + }}$
Answer
578.4k+ views
Hint: Hund’s rule states that electrons arrange in the orbitals in a way that the spin of the electrons remains maximum. The atomic number of Fe, Mn and Cr is 26, 25 and 24 respectively.
Complete Step-by-Step Solution:
We need to compare the magnetic moment of three species given to us. It is given to suppose that Hund’s rule is not followed when the electrons are arranged in the orbitals.
- Hund’s rule states that electrons arrange in the orbitals in a way that the spin of the electrons remains maximum. So, it is also called maximum spin multiplicity rule. Now, we will write the new electronic configuration of given species if Hund’s rule is not followed.
- Electronic configuration of $F{e^{2 + }}$: [Ar]$3{d^6}$
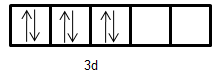
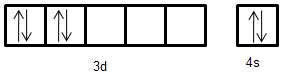
- Electronic configuration of $M{n^ + }$: [Ar]$3{d^5}4{s^1}$

Electronic configuration of Cr: [Ar]$3{d^4}4{s^2}$
- Thus, from the above given electronic configurations we can say that $F{e^{2 + }}$ does not have any unpaired electron. $M{n^ + }$ has two unpaired electrons. Cr atoms also do not have any unpaired electrons.
- We know that the magnetic moment arises from the spin of unpaired electrons.
- So, we can say that the magnetic moment of both $F{e^{2 + }}$ and Cr atoms will be zero. Thus, the magnetic moment of both of them is the same.
- The magnetic moment of $M{n^ + }$ will be some positive value as it has two unpaired electrons.
- Thus, we can say that the order of magnetic moment of these ions will be $F{e^{2 + }} = Cr < M{n^ + }$
So, the correct answer to this question is (B).
Note: Do not forget to arrange the electrons in a way the spin of the electrons remains as less as possible here as Hund’s rule is not followed. Actually, Hund’s rule is followed in nature and $F{e^{2 + }}$ ion has 4, $M{n^ + }$ has 6 and Cr has six unpaired electrons.
Complete Step-by-Step Solution:
We need to compare the magnetic moment of three species given to us. It is given to suppose that Hund’s rule is not followed when the electrons are arranged in the orbitals.
- Hund’s rule states that electrons arrange in the orbitals in a way that the spin of the electrons remains maximum. So, it is also called maximum spin multiplicity rule. Now, we will write the new electronic configuration of given species if Hund’s rule is not followed.
- Electronic configuration of $F{e^{2 + }}$: [Ar]$3{d^6}$

- Electronic configuration of $M{n^ + }$: [Ar]$3{d^5}4{s^1}$

Electronic configuration of Cr: [Ar]$3{d^4}4{s^2}$

- Thus, from the above given electronic configurations we can say that $F{e^{2 + }}$ does not have any unpaired electron. $M{n^ + }$ has two unpaired electrons. Cr atoms also do not have any unpaired electrons.
- We know that the magnetic moment arises from the spin of unpaired electrons.
- So, we can say that the magnetic moment of both $F{e^{2 + }}$ and Cr atoms will be zero. Thus, the magnetic moment of both of them is the same.
- The magnetic moment of $M{n^ + }$ will be some positive value as it has two unpaired electrons.
- Thus, we can say that the order of magnetic moment of these ions will be $F{e^{2 + }} = Cr < M{n^ + }$
So, the correct answer to this question is (B).
Note: Do not forget to arrange the electrons in a way the spin of the electrons remains as less as possible here as Hund’s rule is not followed. Actually, Hund’s rule is followed in nature and $F{e^{2 + }}$ ion has 4, $M{n^ + }$ has 6 and Cr has six unpaired electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




