
IF $ Ca{C_2}\xrightarrow{{Hydrolysis}}A\xrightarrow{{HgS{O_4} + {H_2}S{O_4}}}B $
Then B is :
(A) Acetylene
(B) Acetaldehyde
(C) Acetone
(D) Acetic acid
Answer
502.2k+ views
Hint :Calcium carbide is a compound with carbon and calcium. It undergoes hydrolysis to form acetylene. Further, when acetylene treated with mercuric sulphate and sulphuric acid the acetylene forms enol compounds. This enol compound undergoes tautomerism to form aldehyde known as acetaldehyde.
Complete Step By Step Answer:
Calcium carbide is a chemical compound with the molecular formula of $ Ca{C_2} $ .it contains a calcium ion and two carbon atoms. It is mainly used for the ripening of fruits such as mangoes. But it is not good for your health. Thus, nowadays it was banned.
Hydrolysis is the chemical process of addition of water molecules. Calcium carbide on hydrolysis forms acetylene. Acetylene is an alkyne. It contains two carbon atoms. The molecular formula of acetylene is $ {C_2}{H_2} $ . The structure of acetylene is $ CH \equiv CH $ . Thus, it has a triple bond and is referred to as an unsaturated compound.
Acetylene upon treatment with mercuric sulphate and sulphuric acid forms an enol compound. The enol compound undergoes tautomerism to form a corresponding aldehyde.
Thus, acetylene forms acetaldehyde. Acetaldehyde is a compound with the molecular formula of $ C{H_3}CHO $ . The functional group present is carbonyl group, ketones and aldehyde belong to carbonyl groups.
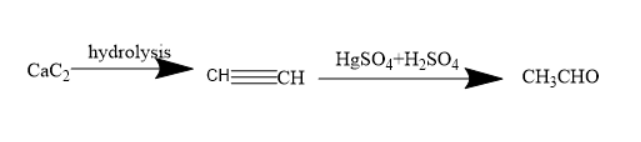
Thus, the compound B is acetaldehyde.
Option B is the correct one.
Note :
The enol compound has a double bond and hydroxyl group. It undergoes tautomerization. The transfer of one hydrogen atom within the compound forms a carbonyl compound aldehyde or ketone. The enol compound forms at first, undergoes tautomerization to form acetaldehyde.
Complete Step By Step Answer:
Calcium carbide is a chemical compound with the molecular formula of $ Ca{C_2} $ .it contains a calcium ion and two carbon atoms. It is mainly used for the ripening of fruits such as mangoes. But it is not good for your health. Thus, nowadays it was banned.
Hydrolysis is the chemical process of addition of water molecules. Calcium carbide on hydrolysis forms acetylene. Acetylene is an alkyne. It contains two carbon atoms. The molecular formula of acetylene is $ {C_2}{H_2} $ . The structure of acetylene is $ CH \equiv CH $ . Thus, it has a triple bond and is referred to as an unsaturated compound.
Acetylene upon treatment with mercuric sulphate and sulphuric acid forms an enol compound. The enol compound undergoes tautomerism to form a corresponding aldehyde.
Thus, acetylene forms acetaldehyde. Acetaldehyde is a compound with the molecular formula of $ C{H_3}CHO $ . The functional group present is carbonyl group, ketones and aldehyde belong to carbonyl groups.

Thus, the compound B is acetaldehyde.
Option B is the correct one.
Note :
The enol compound has a double bond and hydroxyl group. It undergoes tautomerization. The transfer of one hydrogen atom within the compound forms a carbonyl compound aldehyde or ketone. The enol compound forms at first, undergoes tautomerization to form acetaldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




