
If ${C_5}{H_{12}}$ has a symmetrical structure with one quaternary carbon.Than Its IUPAC name is:
A. n-pentane
B. $2$-methylbutane
C. $2,2$-dimethylpropane
D. $3$-methylbutane
Answer
569.4k+ views
Hint: Quaternary carbon will have all its substituents as carbon atoms and the central carbon should not have any hydrogen substituents. For a structure to be symmetrical there should be a plane of symmetry.
Complete step by step answer:
There are 4 types of carbon atoms:
Primary: in these types of carbon atoms there is only one other substituent attached to the carbon atom and the other three valencies out of the total four valencies are occupied by hydrogen.
These are simple alkane chains without any branching.
Secondary: in these types of carbon atoms there are two other substituents attached to the carbon atom and the other two valencies out of the total four valencies are occupied by hydrogen.
These are simple branched alkane chains with only one branched chain
They are of two types:
Iso: this is when the substituent groups are the same on the branched central carbon, usually these are methyl groups but it is not necessary.
Sec: this is when the substituent groups are different on the branched central carbon atom.
Tertiary: in these types of carbon atoms there are three other substituents attached to the carbon atom and the other one valency out of the total four valencies are occupied by hydrogen.
These are alkane chains with two branched chains on a single carbon.
Quaternary this is when all the substituents of the carbon atom are replaced by carbon groups and there are no hydrogen groups. Let us draw the structure of the compounds given to us:
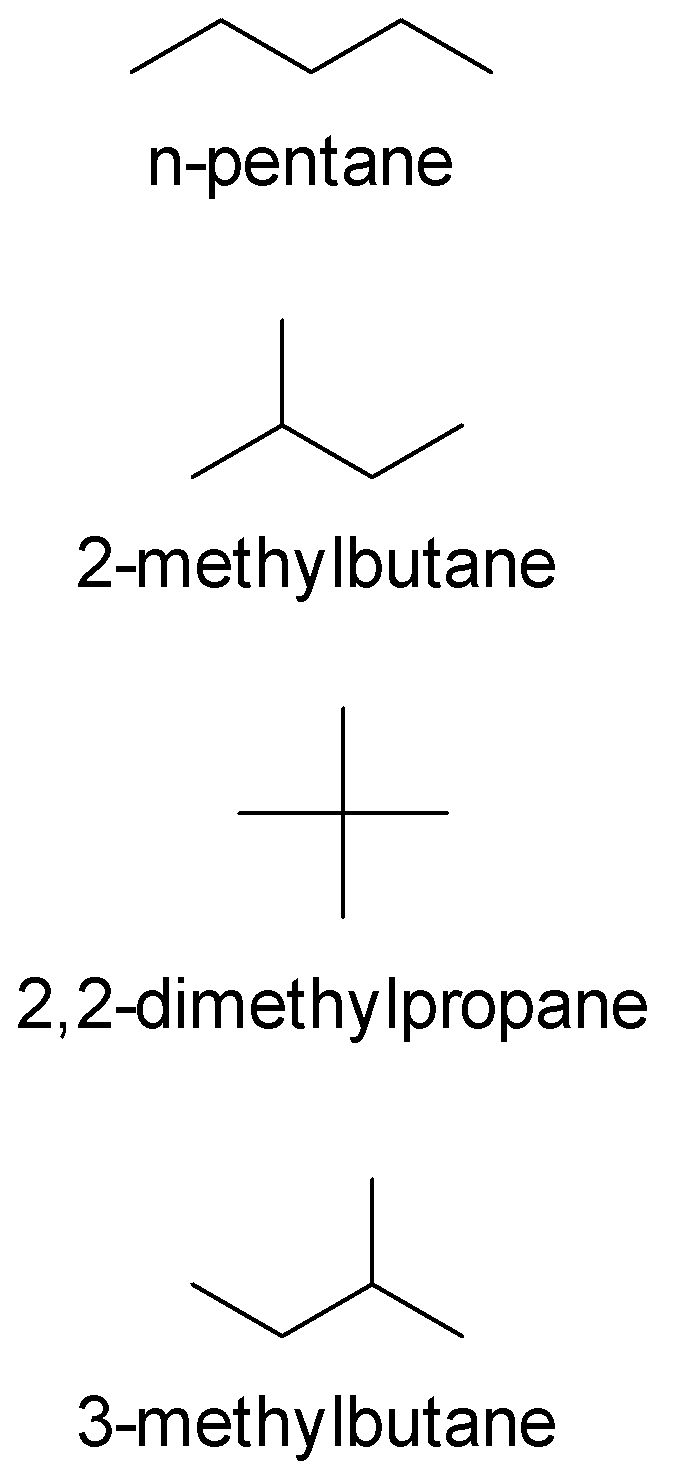
Out of all the four options, the only symmetrical option is $2,2$- dimethylpropane
The central carbon is also a quaternary carbon atom.
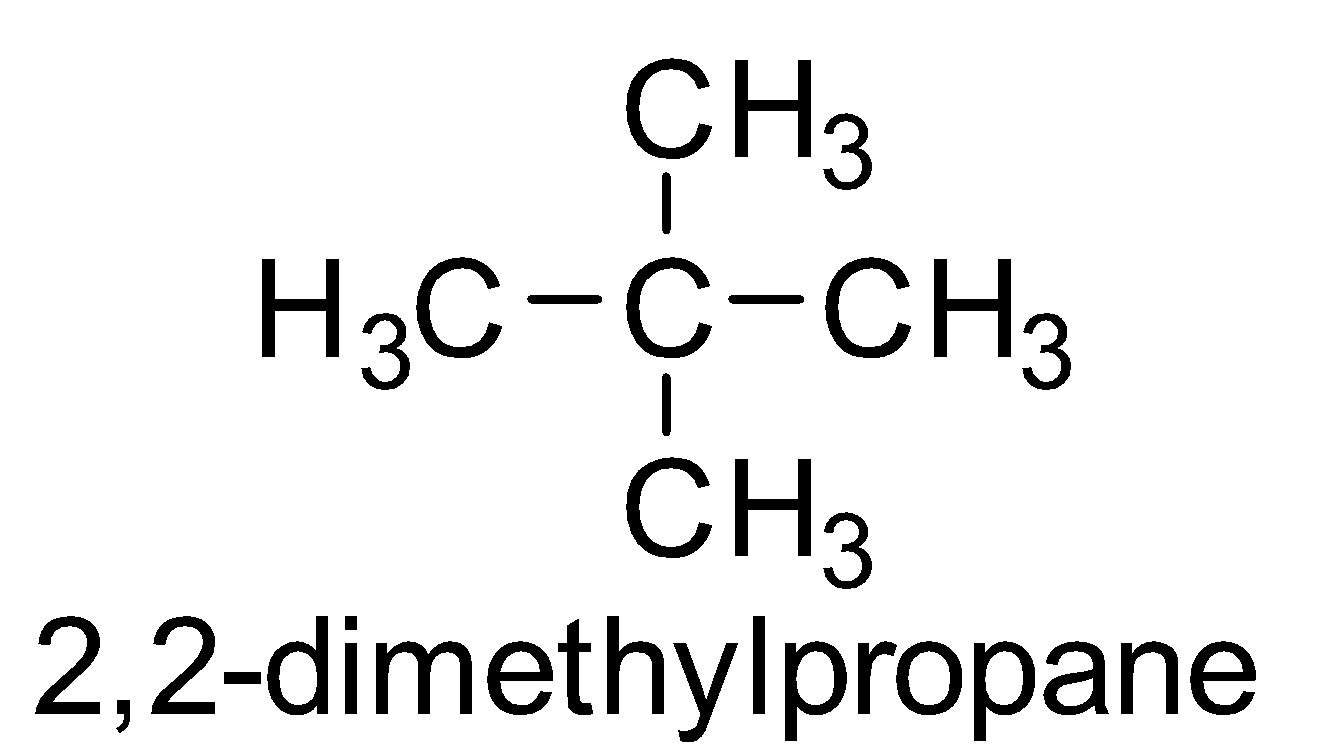
So, the correct answer is Option C.
Note: Compounds with the same molecular formula but different structural formulas are termed as chain isomers . $2,2$- dimethylpropane is a chain isomer of pentane which has a symmetrical structure around the quaternary carbon atom .
Complete step by step answer:
There are 4 types of carbon atoms:
Primary: in these types of carbon atoms there is only one other substituent attached to the carbon atom and the other three valencies out of the total four valencies are occupied by hydrogen.
These are simple alkane chains without any branching.
Secondary: in these types of carbon atoms there are two other substituents attached to the carbon atom and the other two valencies out of the total four valencies are occupied by hydrogen.
These are simple branched alkane chains with only one branched chain
They are of two types:
Iso: this is when the substituent groups are the same on the branched central carbon, usually these are methyl groups but it is not necessary.
Sec: this is when the substituent groups are different on the branched central carbon atom.
Tertiary: in these types of carbon atoms there are three other substituents attached to the carbon atom and the other one valency out of the total four valencies are occupied by hydrogen.
These are alkane chains with two branched chains on a single carbon.
Quaternary this is when all the substituents of the carbon atom are replaced by carbon groups and there are no hydrogen groups. Let us draw the structure of the compounds given to us:

Out of all the four options, the only symmetrical option is $2,2$- dimethylpropane
The central carbon is also a quaternary carbon atom.

So, the correct answer is Option C.
Note: Compounds with the same molecular formula but different structural formulas are termed as chain isomers . $2,2$- dimethylpropane is a chain isomer of pentane which has a symmetrical structure around the quaternary carbon atom .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




