
If an atom has electronic configuration $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^3}4{s^2}$, it will be placed in
A. Second group
B. Third group
C. Fifth group
D. Sixth group
Answer
570k+ views
Hint: All the elements in the group have the same outer shell configuration. The periodic table can also be divided into blocks, such as the s block, p block, d block and f block. Elements are divided into these blocks on the basis of in which shell does the incoming electron go.
Complete step by step answer:
The periodic table is organised in such a way, that elements with the same outer shell configuration will fall in the same group.
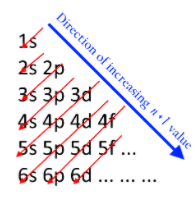
For example, all the elements in group one will have the outer shell electronic configuration as $n{s^1},n = period\,number$ Elements in first group are $Na,K$ $Na = 1{s^1}2{s^2}2{p^6}3{s^1}$
$K = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}$
Notice that the last electron in both the elements goes into the s shell and the s shell is half filled with only one electron. The periodic table is divided as: $s\,block = 1 - 2group$
$d\,block = 3 - 12block$
$p\,block = 13 - 18block$
According to the Aufbau principle the of $4s$orbital is less than that of $3d$ orbital and hence the $4s$ orbital is filled first, but while writing the electronic configuration $3d$ is mentioned before $4s$ . Hence, The outermost electronic configuration for elements in the d block goes like, $(n - 1){d^{1 - 10}}n{s^2}$. The d electrons have shell number one less than the s shell number. So, in the configuration $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^3}4{s^2}$, the last shell to be filled is the d shell and the its configuration is $3{d^3}$. This means that the incoming electron goes to the d block and hence, the elements must belong to the d block in the periodic table. The general configuration of the elements is, $(n - 1){d^1}n{s^2}$, we will add the value of the outermost electron to get the value of shell number. $\therefore 1 + 2 = 3$ Hence, Elements with outer shell electronic configuration as $(n - 1){d^1}n{s^2}$ will be in the third group. In our question, the outer shell electronic configuration is $3{d^3}4{s^2}$. By adding the value of the outermost shell electrons we get $3 + 2 = 5$ Hence, this element belongs to the fifth group.
So, the correct answer is “Option C”.
Note: The d block elements are also known as transition metal, and they have a separate group called as inner transition metals or the f block elements. These elements are mostly the radioactive materials found in the periodic table.
The metal with electronic configuration: $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^3}4{s^2}$ is Chromium with atomic number $23$ .
Transition materials have the most wide range applications out of any other group of elements.If the outermost electrons are in the p orbital, for example if the element has three electrons in the p orbital and ten electrons in the d orbital, then it goes in the thirteenth group. But if it only has electrons in the d orbital and not the p, it goes in the group number which will be the sum of s and d orbital.
Complete step by step answer:
The periodic table is organised in such a way, that elements with the same outer shell configuration will fall in the same group.

For example, all the elements in group one will have the outer shell electronic configuration as $n{s^1},n = period\,number$ Elements in first group are $Na,K$ $Na = 1{s^1}2{s^2}2{p^6}3{s^1}$
$K = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}$
Notice that the last electron in both the elements goes into the s shell and the s shell is half filled with only one electron. The periodic table is divided as: $s\,block = 1 - 2group$
$d\,block = 3 - 12block$
$p\,block = 13 - 18block$
According to the Aufbau principle the of $4s$orbital is less than that of $3d$ orbital and hence the $4s$ orbital is filled first, but while writing the electronic configuration $3d$ is mentioned before $4s$ . Hence, The outermost electronic configuration for elements in the d block goes like, $(n - 1){d^{1 - 10}}n{s^2}$. The d electrons have shell number one less than the s shell number. So, in the configuration $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^3}4{s^2}$, the last shell to be filled is the d shell and the its configuration is $3{d^3}$. This means that the incoming electron goes to the d block and hence, the elements must belong to the d block in the periodic table. The general configuration of the elements is, $(n - 1){d^1}n{s^2}$, we will add the value of the outermost electron to get the value of shell number. $\therefore 1 + 2 = 3$ Hence, Elements with outer shell electronic configuration as $(n - 1){d^1}n{s^2}$ will be in the third group. In our question, the outer shell electronic configuration is $3{d^3}4{s^2}$. By adding the value of the outermost shell electrons we get $3 + 2 = 5$ Hence, this element belongs to the fifth group.
So, the correct answer is “Option C”.
Note: The d block elements are also known as transition metal, and they have a separate group called as inner transition metals or the f block elements. These elements are mostly the radioactive materials found in the periodic table.
The metal with electronic configuration: $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^3}4{s^2}$ is Chromium with atomic number $23$ .
Transition materials have the most wide range applications out of any other group of elements.If the outermost electrons are in the p orbital, for example if the element has three electrons in the p orbital and ten electrons in the d orbital, then it goes in the thirteenth group. But if it only has electrons in the d orbital and not the p, it goes in the group number which will be the sum of s and d orbital.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




