
Identify A and predict the type of reaction.
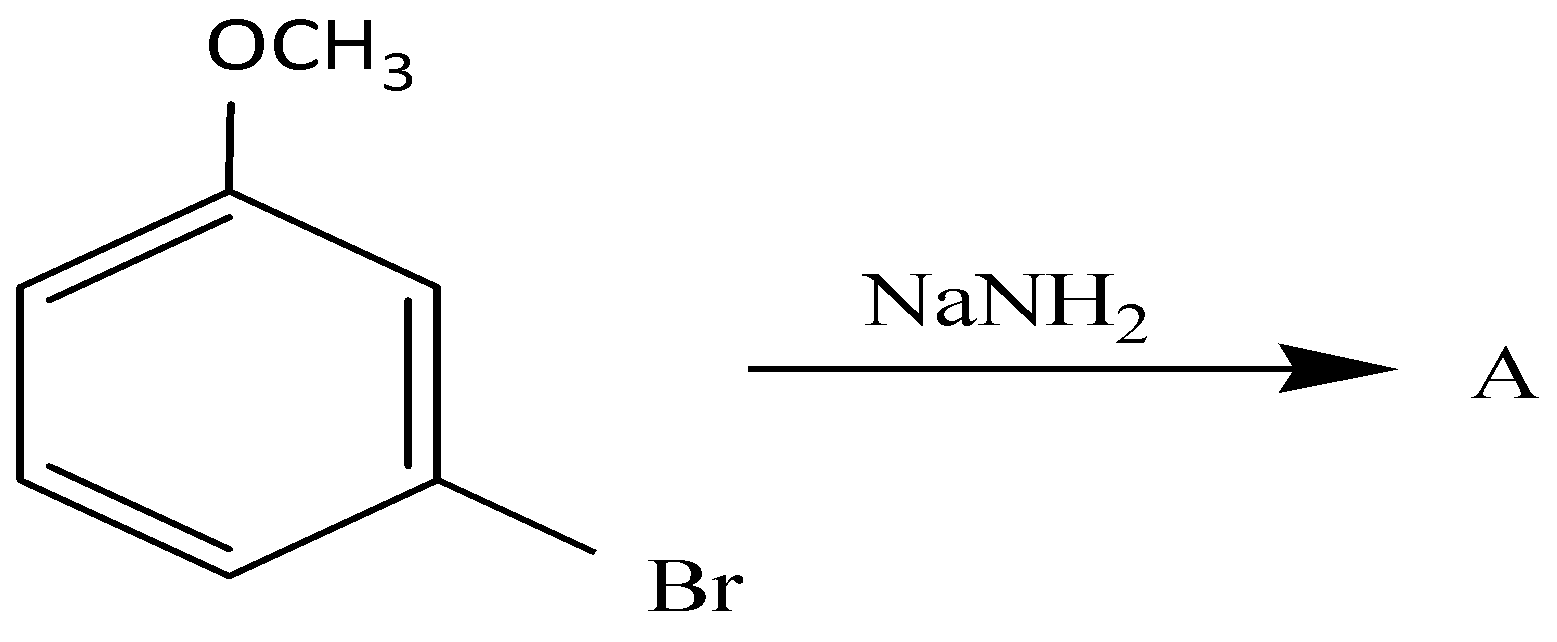
(A)
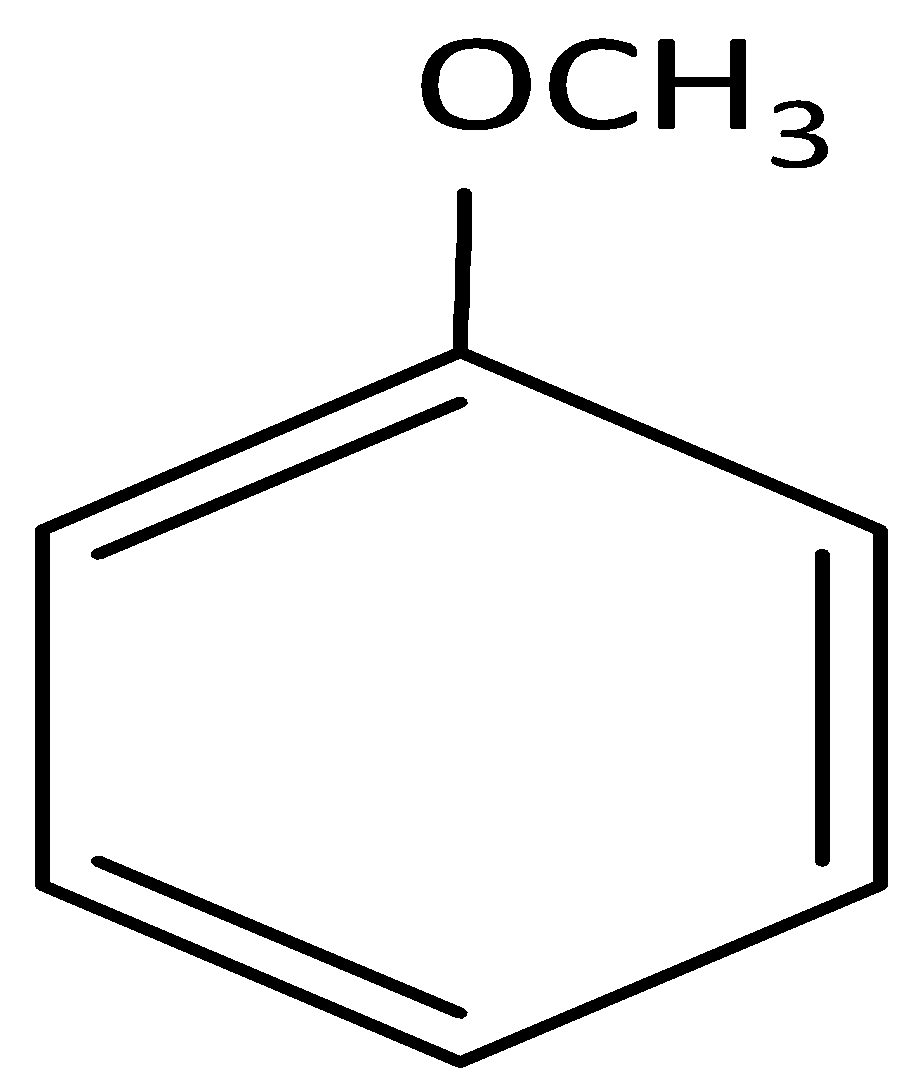 and cine substitution reaction
and cine substitution reaction
(B)
 and substitution reaction
and substitution reaction
(C)
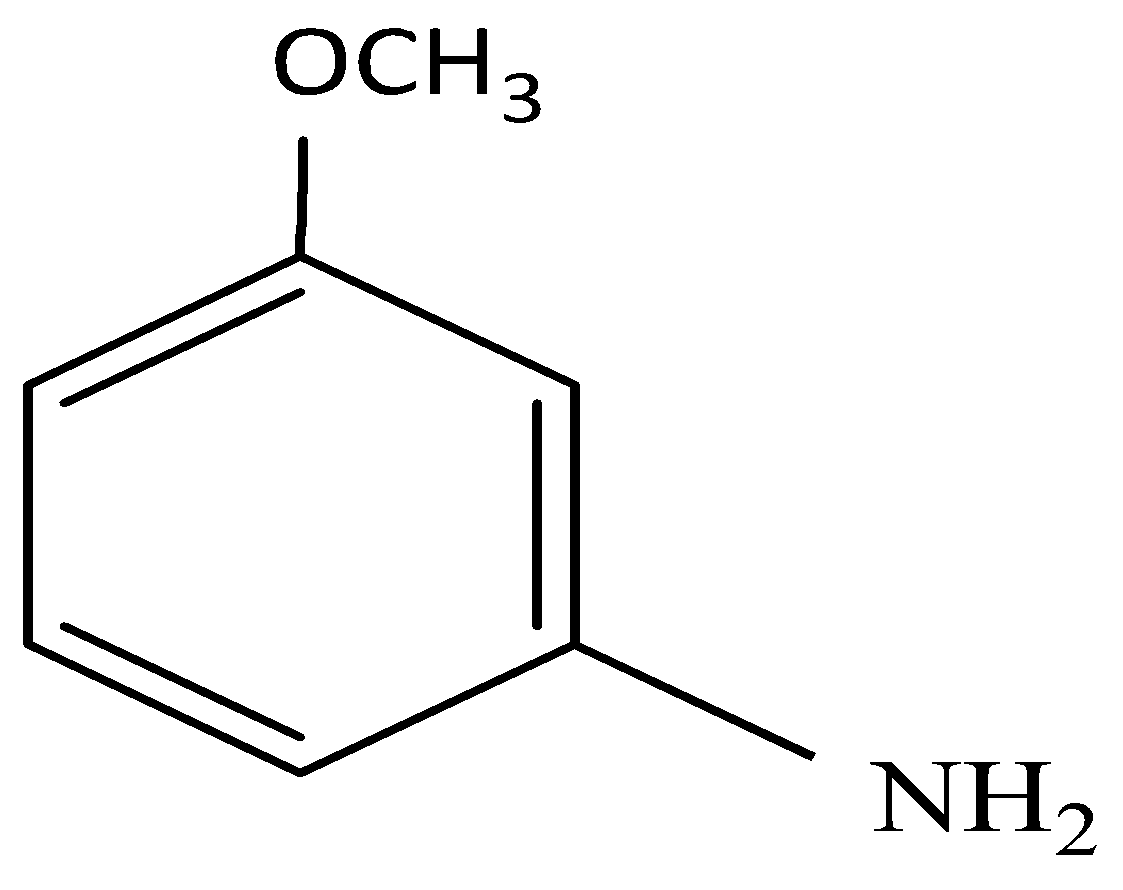 and elimination addition reaction
and elimination addition reaction
(D)
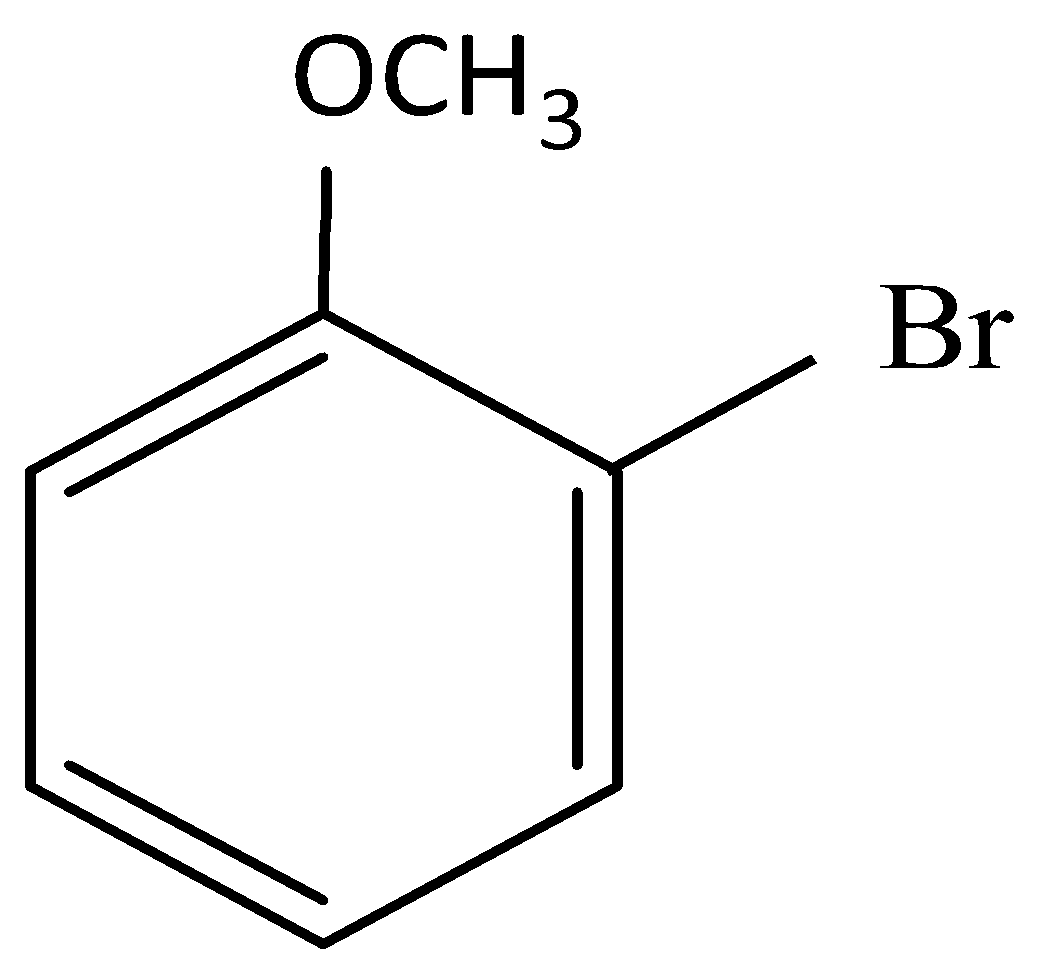 and cine substitution reaction
and cine substitution reaction





Answer
569.4k+ views
Hint: We need to know what is a nucleophile and an electrophile and whether the given compound used in the reaction (\[NaN{H_2}\]) is a nucleophile or an electrophile. Electrophile and nucleophile is a chemical species that donate or accept electrons to form a new chemical bond. A nucleophile is usually charged negatively or neutral with a lone couple of donable electrons. Positively loaded or neutral species are called electrophiles that are deficient in electrons and can accept a couple of electrons.
Complete step by step answer:
We have to remember that the \[NaN{H_2}\] is also known as sodium amide or sodamide. It is a strong base capable of deprotonating.
In the given reaction, \[NaN{H_2}\] acts as a nucleophile and the following takes place:
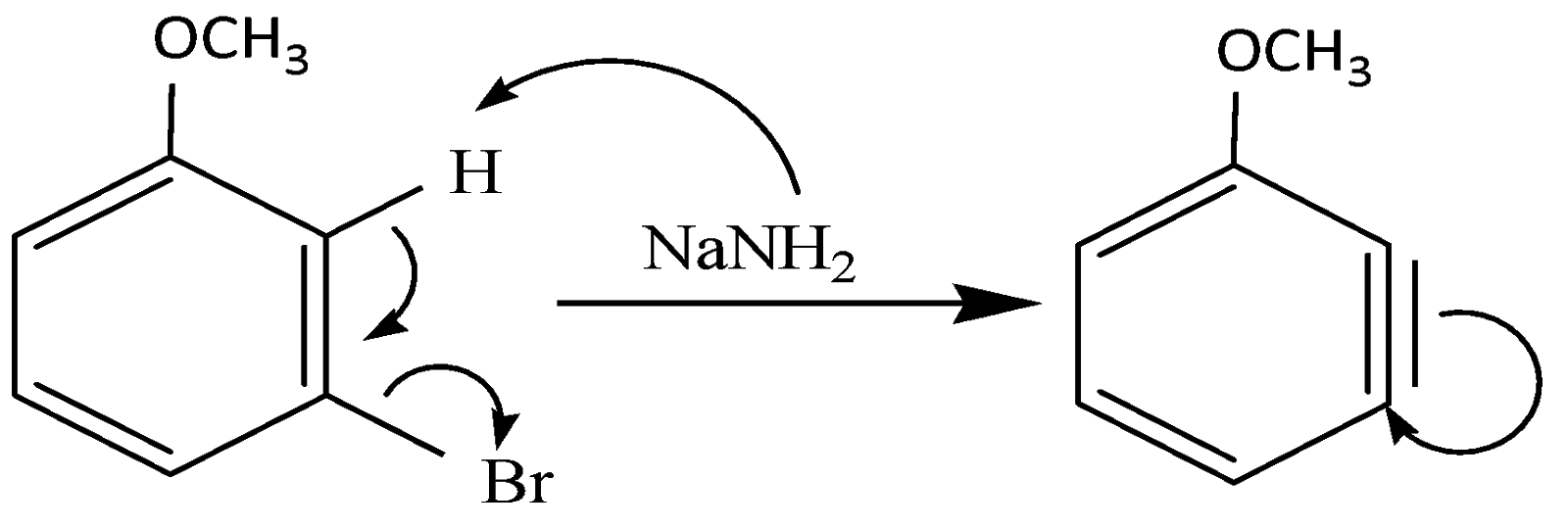
(i) The \[N{H_2}\] deprotonates the \[H\] group and as a result it gives its electron to the benzene ring and \[Br\] acts as a leaving group and leaves behind a benzyne intermediate. This intermediate is formed as a result of elimination reaction.
(ii) The triple bond breaks and donates its hydrogen to the incoming \[N{H_2}\] group and the resulting molecule is:
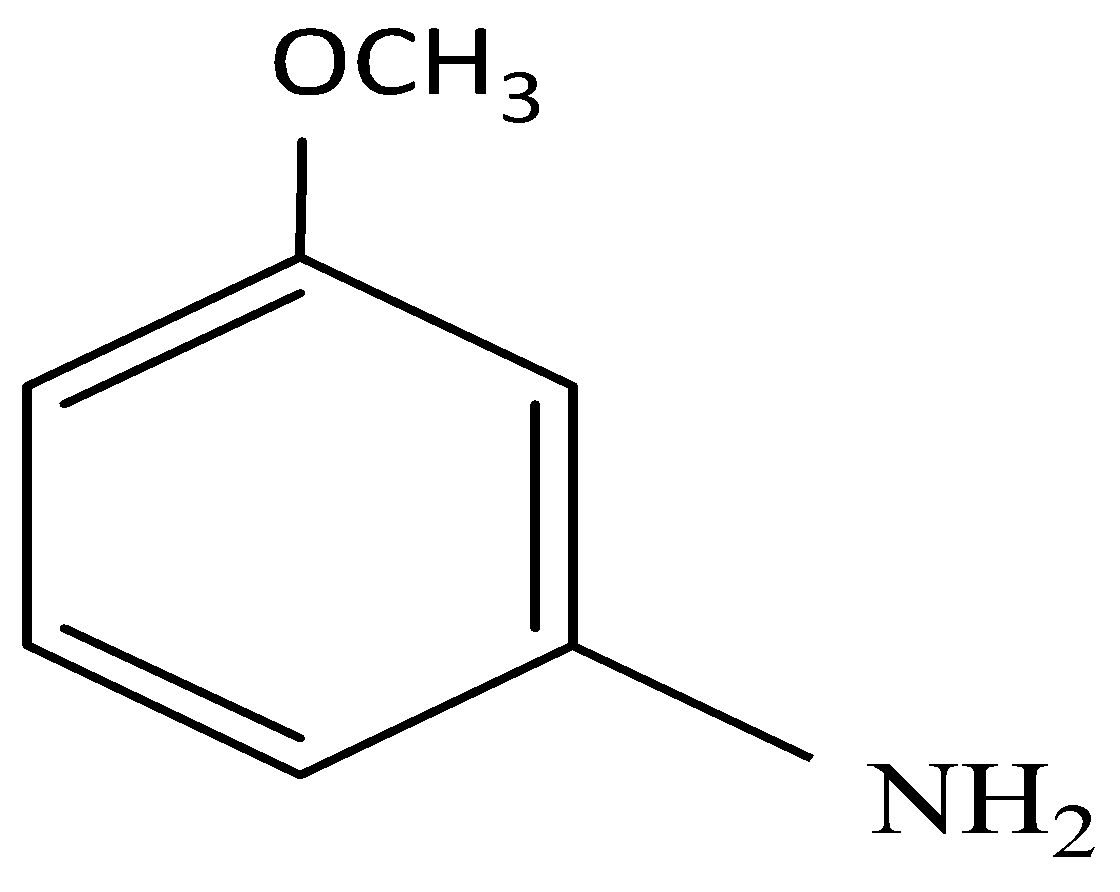
It is clear that elimination of \[Br\] is followed by addition of \[N{H_2}\].
So, the correct answer is Option C.
Note: It must be noted that in order to predict the product of such reactions, the nature of the attacking group must be known, i.e. whether the attacking group is a nucleophile or an electrophile. The difference between the two must be clear. Also, nucleophilic elimination addition reaction is also known as nucleophilic aromatic substitution reaction. It is also important to know the difference between a substitution reaction and cine substitution reaction. Cine- substitution is a substitution reaction (generally aromatic) in which the entering group takes up a position adjacent to that occupied by the leaving group.
Complete step by step answer:
We have to remember that the \[NaN{H_2}\] is also known as sodium amide or sodamide. It is a strong base capable of deprotonating.
In the given reaction, \[NaN{H_2}\] acts as a nucleophile and the following takes place:

(i) The \[N{H_2}\] deprotonates the \[H\] group and as a result it gives its electron to the benzene ring and \[Br\] acts as a leaving group and leaves behind a benzyne intermediate. This intermediate is formed as a result of elimination reaction.
(ii) The triple bond breaks and donates its hydrogen to the incoming \[N{H_2}\] group and the resulting molecule is:

It is clear that elimination of \[Br\] is followed by addition of \[N{H_2}\].
So, the correct answer is Option C.
Note: It must be noted that in order to predict the product of such reactions, the nature of the attacking group must be known, i.e. whether the attacking group is a nucleophile or an electrophile. The difference between the two must be clear. Also, nucleophilic elimination addition reaction is also known as nucleophilic aromatic substitution reaction. It is also important to know the difference between a substitution reaction and cine substitution reaction. Cine- substitution is a substitution reaction (generally aromatic) in which the entering group takes up a position adjacent to that occupied by the leaving group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




