
How would you identify which carbon is sp, \[s{{p}^{2}}\] and \[s{{p}^{3}}\] ?
Answer
533.7k+ views
Hint: The hybridisation is the concept of the mixing of the two orbitals of atoms which has the same energy levels so that they give a degenerated new type of the orbitals. This concept of the intermixing of the atomic orbitals is based on the concept of quantum mechanics. The condition for mixing of two orbitals is that both the orbitals of the atom should have the same energy levels and can be half filled or fully filled.
Complete step by step answer:
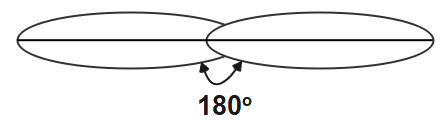
The first type of hybridisation is sp hybridisation. We can identify the sp hybridised carbon atom if the s orbital and the p orbital in the same main shell of the atom is been mixed to form the two new equivalent orbitals. They sp hybridised carbon atom will form the linear molecule with the angle of \[{{180}^{o}}\] . It is also known as diagonal hybridisation. In this sp hybridisation the equal amount of s and p character is present that is \[50%\] each.
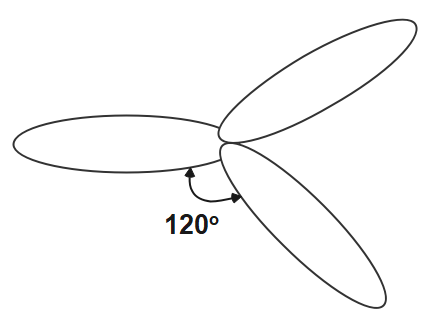
The other hybridisation is \[s{{p}^{2}}\] in which we see that one s orbital and two orbitals of p of the same shell of the atom get mix to form the $3$ equivalent orbitals. These three orbitals are in one plane thus making the angle of \[{{120}^{o}}\] . The s character in this hybridisation is \[33.33%\] and p character is \[66.66%.\]
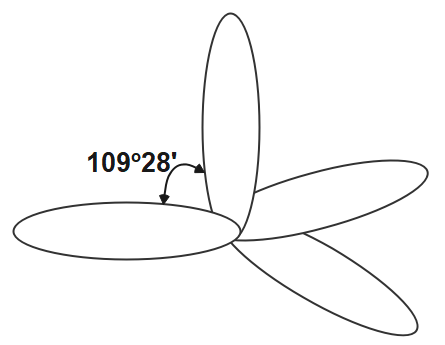
The other hybridisation is \[s{{p}^{3}}\] hybridisation. In this hybridisation one orbital of s and three orbitals of p of the same shell of the atom get mixed to form the four new equivalent orbits. In this orbitals the angle which formed between the orbitals is \[{{109}^{o}}{{28}^{'}}\] . the s character in this hybridisation is \[25%\] and p character is \[75%.\]
So if the carbon is \[s{{p}^{3}}\] hybridised it will form all single bonds. But the carbon is \[s{{p}^{2}}\] hybridised if it forms a double bond. The carbon is sp hybridised if it forms a triple bond. Look at the following examples
\[C{{H}_{4}}\] (methane)- in this the carbon is \[s{{p}^{3}}\] because it forms four single bonds with the hydrogen atoms.
\[C{{H}_{3}}=CH-C{{H}_{3}}\] - here the second carbon is \[s{{p}^{2}}\] hybridised as it forms double bonds.
\[C{{H}_{3}}=C-C{{H}_{3}}\] - here the second carbon is sp hybridised as it forms a triple bond.
Note: The orbitals of atoms with the same energies undergo hybridisation. The number of the hybrid orbitals formed is equal to the number of the orbitals of atom mixed. The hybridisation process occurs during the bond formation.
Complete step by step answer:
The first type of hybridisation is sp hybridisation. We can identify the sp hybridised carbon atom if the s orbital and the p orbital in the same main shell of the atom is been mixed to form the two new equivalent orbitals. They sp hybridised carbon atom will form the linear molecule with the angle of \[{{180}^{o}}\] . It is also known as diagonal hybridisation. In this sp hybridisation the equal amount of s and p character is present that is \[50%\] each.

The other hybridisation is \[s{{p}^{2}}\] in which we see that one s orbital and two orbitals of p of the same shell of the atom get mix to form the $3$ equivalent orbitals. These three orbitals are in one plane thus making the angle of \[{{120}^{o}}\] . The s character in this hybridisation is \[33.33%\] and p character is \[66.66%.\]

The other hybridisation is \[s{{p}^{3}}\] hybridisation. In this hybridisation one orbital of s and three orbitals of p of the same shell of the atom get mixed to form the four new equivalent orbits. In this orbitals the angle which formed between the orbitals is \[{{109}^{o}}{{28}^{'}}\] . the s character in this hybridisation is \[25%\] and p character is \[75%.\]

So if the carbon is \[s{{p}^{3}}\] hybridised it will form all single bonds. But the carbon is \[s{{p}^{2}}\] hybridised if it forms a double bond. The carbon is sp hybridised if it forms a triple bond. Look at the following examples
\[C{{H}_{4}}\] (methane)- in this the carbon is \[s{{p}^{3}}\] because it forms four single bonds with the hydrogen atoms.
\[C{{H}_{3}}=CH-C{{H}_{3}}\] - here the second carbon is \[s{{p}^{2}}\] hybridised as it forms double bonds.
\[C{{H}_{3}}=C-C{{H}_{3}}\] - here the second carbon is sp hybridised as it forms a triple bond.
Note: The orbitals of atoms with the same energies undergo hybridisation. The number of the hybrid orbitals formed is equal to the number of the orbitals of atom mixed. The hybridisation process occurs during the bond formation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




