
Identify the true statement regarding Daniel cell:
A) Zinc ions flow across salt bridge
B) $\mathrm{K}^{+}$ ions move from salt bridge to $\mathrm{Cu} / \mathrm{Cu}^{2+}$ half cell
C) Oxidation takes place at copper electrode
D) Flow of current takes place from copper electrode to zinc electrode
Answer
585.6k+ views
Hint: Daniell cell is a type of cell that is electrochemical in nature. It consists of a copper pot filled with copper(II) sulphate solution, sulphuric acid and zinc electrode.
Complete step by step answer:
In the wet-cell during discharge, nitrate anions in the salt bridge move into the zinc half-cell in order to balance the increase in $\mathrm{Zn}^{2+}$ ions. At the same time, potassium ions from the salt bridge move into the copper half-cell in order to replace the $\mathrm{Cu}^{2+}$ ions being precipitated onto the copper electrode.
At anode:
$\mathrm{Zn}_{(\mathrm{s})} \rightarrow \mathrm{Zn}_{(\mathrm{aq})}^{2+}+2 \mathrm{e}^{-}$
At cathode:
$\mathrm{Cu}_{(\mathrm{aq})}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}_{(\mathrm{s})}$
The total reaction is:
$\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})$
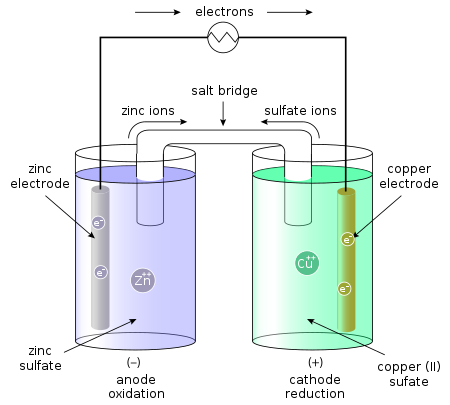
The Daniell cell performs the spontaneous redox reaction between zinc and cupric ions to
produce an electric current. It consists of two half-cells. The left half cell contains a zinc metal
electrode ZnSO dipped in solution.The half right half cell consists of copper metal electrode in
a solution $\mathrm{CuSO}_{4}$ solution.The half-cells are joined by a salt bridge that prevents the mixing of the solution.
In Daniel cell flow of current takes place from copper electrode to zinc electrode.
So, the correct answer is “Option D”.
Note: Daniell cell is an electrochemical cell. Do not confuse this with electrolytic cells. Electrolytic cell is a different cell, in which the electrical energy is converted into chemical energy.
Complete step by step answer:
In the wet-cell during discharge, nitrate anions in the salt bridge move into the zinc half-cell in order to balance the increase in $\mathrm{Zn}^{2+}$ ions. At the same time, potassium ions from the salt bridge move into the copper half-cell in order to replace the $\mathrm{Cu}^{2+}$ ions being precipitated onto the copper electrode.
At anode:
$\mathrm{Zn}_{(\mathrm{s})} \rightarrow \mathrm{Zn}_{(\mathrm{aq})}^{2+}+2 \mathrm{e}^{-}$
At cathode:
$\mathrm{Cu}_{(\mathrm{aq})}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}_{(\mathrm{s})}$
The total reaction is:
$\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})$

The Daniell cell performs the spontaneous redox reaction between zinc and cupric ions to
produce an electric current. It consists of two half-cells. The left half cell contains a zinc metal
electrode ZnSO dipped in solution.The half right half cell consists of copper metal electrode in
a solution $\mathrm{CuSO}_{4}$ solution.The half-cells are joined by a salt bridge that prevents the mixing of the solution.
In Daniel cell flow of current takes place from copper electrode to zinc electrode.
So, the correct answer is “Option D”.
Note: Daniell cell is an electrochemical cell. Do not confuse this with electrolytic cells. Electrolytic cell is a different cell, in which the electrical energy is converted into chemical energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




