
Identify the total number of compounds which are unstable at room temperature.
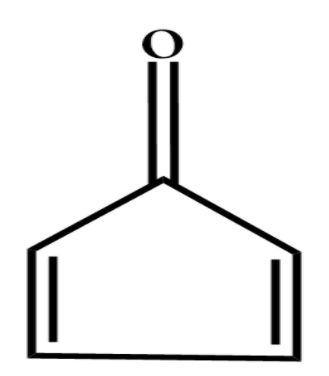
(A)
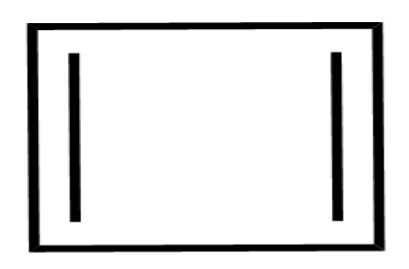
(B)
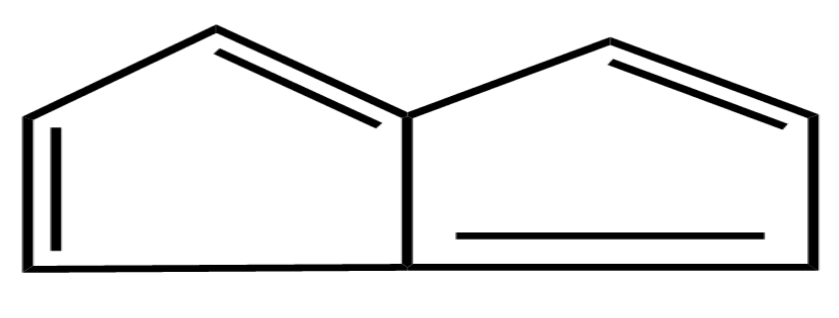
(C)
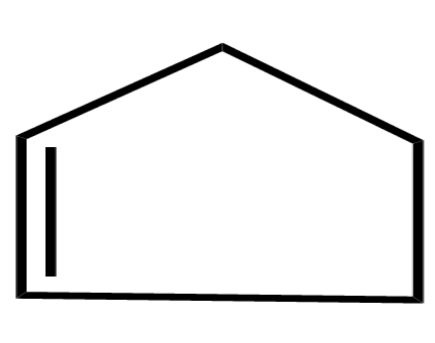
(D)




Answer
510.6k+ views
Hint: We will study each option and determine which compound is least stable among them. We will use the Huckel Rule which says if a compound contains $ 4n(n = 0,1,2,3....) $ delocalised $ \pi $ electrons in a closed loop, then it is considered as Anti-Aromatic and if a cyclic, planar compound contains $ 4n + 2(n = 0,1,2,3...) $ $ \pi $ electrons it is said to be Aromatic compounds. Compounds that do not satisfy the above 2 conditions are known as non-Aromatic Compounds. We have an order of stability between Aromatic, Anti-Aromatic, non-Aromatic compounds.
$ Aromatic > Non - Aromatic > Anti - Aromatic $
Complete answer:
Let’s study the first option, this compound is known as Cyclobutadiene. This compound is cyclic and contains $ 4\pi $ electrons, now according to the Huckel Rule the cyclic compound that contains $ 4n(n = 0,1,2,3....) $ $ \pi $ electrons is Antiaromatic.
The compound in the second option contains $ 8\pi $ electrons but it does not have delocalised electrons so resonance is not possible in this compound. so, this compound is non-Aromatic.
Let’s see the third option the compound is known as Cyclopentene it contains $ 2\pi $ electrons and it is an alicyclic compound it does not have cyclic structure and contains only single double bond so, it is non-Aromatic
Now, the last option remains: this compound is not cyclic and due to its bonding, it is not planar as well. Resonance cannot be achieved in this compound so as it is neither cyclic nor planar so it is not aromatic and also not anti-aromatic, it is a non-aromatic compound.
Besides the first compound all other compounds are non-aromatic and by checking the stability order of aromatic non aromatic and antiaromatic compounds we found out that anti-aromatic compounds are the least stable compounds.
So, the correct option is option (A).
Note:
It is important to satisfy all conditions of Huckel Rule for an compound to be aromatic must be cyclic, planar and have $ 4n + 2(n = 0,1,2,3...) $ $ \pi $ electrons, for anti-aromatic compound must be cyclic and contain $ 4n(n = 0,1,2,3...) $ $ \pi $ electrons.
$ Aromatic > Non - Aromatic > Anti - Aromatic $
Complete answer:
Let’s study the first option, this compound is known as Cyclobutadiene. This compound is cyclic and contains $ 4\pi $ electrons, now according to the Huckel Rule the cyclic compound that contains $ 4n(n = 0,1,2,3....) $ $ \pi $ electrons is Antiaromatic.
The compound in the second option contains $ 8\pi $ electrons but it does not have delocalised electrons so resonance is not possible in this compound. so, this compound is non-Aromatic.
Let’s see the third option the compound is known as Cyclopentene it contains $ 2\pi $ electrons and it is an alicyclic compound it does not have cyclic structure and contains only single double bond so, it is non-Aromatic
Now, the last option remains: this compound is not cyclic and due to its bonding, it is not planar as well. Resonance cannot be achieved in this compound so as it is neither cyclic nor planar so it is not aromatic and also not anti-aromatic, it is a non-aromatic compound.
Besides the first compound all other compounds are non-aromatic and by checking the stability order of aromatic non aromatic and antiaromatic compounds we found out that anti-aromatic compounds are the least stable compounds.
So, the correct option is option (A).
Note:
It is important to satisfy all conditions of Huckel Rule for an compound to be aromatic must be cyclic, planar and have $ 4n + 2(n = 0,1,2,3...) $ $ \pi $ electrons, for anti-aromatic compound must be cyclic and contain $ 4n(n = 0,1,2,3...) $ $ \pi $ electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




