
Identify the structure of \[SnC{{l}_{2}}.\]
Answer
524.1k+ views
Hint: We know that in solid form, \[SnC{{l}_{2}}\] is a crystalline mass whose chemical name is Tin \[\left( II \right)\] chloride. Some other names of Tin \[\left( II \right)\]chloride are Tin dichloride, or Dichlorotin, or Tin Proto Chloride, or Stannous chloride. It has a lone pair of electrons where the molecule in the gaseous phase is bent.
Complete answer:
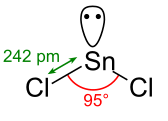
The structure of $SnCl_2$ is a trigonal pyramidal shape or we can say that V shape due to the presence of a lone pair of electrons based on VSEPR theory. [Image will be uploaded soon] The exact mass and the mono-isotopic mass of Tin dichloride is \[189.84\text{ }g/mol.\]The number of hydrogen bond acceptors and the number of hydrogen bond donors equals to zero.
This compound is canonicalized and has one covalently bonded unit only. In chemistry, VSEPR Theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. This theory is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized.
The VSEPR theory is based on the principle that there is a repulsion between the pairs of valence electrons in all atoms. The arrangement of atoms will always be in such a manner in which this electron pair repulsion is minimalized. With the help of this arrangement of the atom, we determine the geometry of the resulting molecule.
In the Trigonal Planar Shape of the molecule, we find three molecules attached to a central atom. Atoms are arranged in such a manner that repulsion between the electrons can be minimized towards the corners of an equilateral triangle. The structure is given as: \[\underset{\bullet \bullet }{\overset{\bullet \bullet }{\mathop{:Cl}}}\,-\underset{{}}{\overset{\bullet \bullet }{\mathop{Sn}}}\,-\underset{\bullet \bullet }{\overset{\bullet \bullet }{\mathop{Cl}}}\,:\]
Note:
Remember that there is a harmful effect of \[SnC{{l}_{2}}\] on our health. Tin Proto Chloride is toxic and corrosive in nature but it is a non-combustible compound. Inhaling, swallowing, or skin contact with this compound can cause severe injuries or lead to death. In its molten form, it may result in severe burns on the skin and in the eyes. When heated, it liberates corrosive, irritating, and toxic gases.
Complete answer:
The structure of $SnCl_2$ is a trigonal pyramidal shape or we can say that V shape due to the presence of a lone pair of electrons based on VSEPR theory. [Image will be uploaded soon] The exact mass and the mono-isotopic mass of Tin dichloride is \[189.84\text{ }g/mol.\]The number of hydrogen bond acceptors and the number of hydrogen bond donors equals to zero.
This compound is canonicalized and has one covalently bonded unit only. In chemistry, VSEPR Theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. This theory is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized.
The VSEPR theory is based on the principle that there is a repulsion between the pairs of valence electrons in all atoms. The arrangement of atoms will always be in such a manner in which this electron pair repulsion is minimalized. With the help of this arrangement of the atom, we determine the geometry of the resulting molecule.
In the Trigonal Planar Shape of the molecule, we find three molecules attached to a central atom. Atoms are arranged in such a manner that repulsion between the electrons can be minimized towards the corners of an equilateral triangle. The structure is given as: \[\underset{\bullet \bullet }{\overset{\bullet \bullet }{\mathop{:Cl}}}\,-\underset{{}}{\overset{\bullet \bullet }{\mathop{Sn}}}\,-\underset{\bullet \bullet }{\overset{\bullet \bullet }{\mathop{Cl}}}\,:\]

Note:
Remember that there is a harmful effect of \[SnC{{l}_{2}}\] on our health. Tin Proto Chloride is toxic and corrosive in nature but it is a non-combustible compound. Inhaling, swallowing, or skin contact with this compound can cause severe injuries or lead to death. In its molten form, it may result in severe burns on the skin and in the eyes. When heated, it liberates corrosive, irritating, and toxic gases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




