
Identify the products of the reaction:
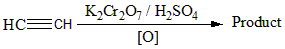
A) $C{H_3}CHO$
B) $C{H_3}C{H_2}OH$
C) $C{H_3}COOH$
D) $C{H_3}OH$
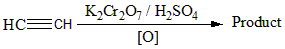
Answer
497.4k+ views
Hint: The given reactant to us is Acetylene. It is an alkyne with two carbon atoms and a triple bond. It has a formula ${C_2}{H_2}$ . The reagent given to us is a strong oxidising agent Potassium Dichromate. Potassium Dichromate on reaction with Sulphuric acid, gives nascent oxygen which causes oxidation.
Complete answer:
Acetylene, in reaction with Strong oxidising agent like Potassium dichromate, converts it into acetic acid. But note that this reaction cannot occur directly. First, we’ll have to change the Acetylene into Aldehyde and then on further oxidation it changes into carboxylic acid.
Conversion of acetylene into aldehyde occurs in presence of $40\% {H_2}S{O_4}$ and $1\% HgS{O_4}$ at ${60^ \circ }C$. The reaction for the conversion of Acetylene into aldehyde can be given as:
${C_2}{H_2}\xrightarrow[{HgS{O_4}}]{{{H_2}S{O_4}}}C{H_3}CHO$
Here, acetylene gets converted into acetaldehyde. Acetaldehyde is a two-carbon aldehyde, has a Carbonyl Group and no other C-C multiple bonds present.
Further on reaction with Oxidising agents like Potassium Dichromate, it gets converted into acetic acid.
$C{H_3}CHO\xrightarrow{{[O]}}C{H_3}COOH$
The overall reaction can be shown as:
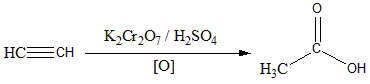
Hence the correct answer is Option (C)
Option (A) would be incorrect because Potassium Dichromate is a strong oxidising agent, and the reaction will proceed further until no further oxidation can take place. Hence it will form the most oxidised product, which in this case is the Carboxylic acid. If mild oxidising agents like PCC were used, then the reaction would stop at the formation of the first oxidised product; aldehyde.
Hence the correct answer is Option (C).
Note:
Acetylene and acetic acid are the common names known for these compounds. The IUPAC names of these compounds are Ethyne (acetylene) and Ethanoic Acid (acetic acid). The major use of acetic acid in households is in the form of Vinegar, which is the diluted form of it.
Complete answer:
Acetylene, in reaction with Strong oxidising agent like Potassium dichromate, converts it into acetic acid. But note that this reaction cannot occur directly. First, we’ll have to change the Acetylene into Aldehyde and then on further oxidation it changes into carboxylic acid.
Conversion of acetylene into aldehyde occurs in presence of $40\% {H_2}S{O_4}$ and $1\% HgS{O_4}$ at ${60^ \circ }C$. The reaction for the conversion of Acetylene into aldehyde can be given as:
${C_2}{H_2}\xrightarrow[{HgS{O_4}}]{{{H_2}S{O_4}}}C{H_3}CHO$
Here, acetylene gets converted into acetaldehyde. Acetaldehyde is a two-carbon aldehyde, has a Carbonyl Group and no other C-C multiple bonds present.
Further on reaction with Oxidising agents like Potassium Dichromate, it gets converted into acetic acid.
$C{H_3}CHO\xrightarrow{{[O]}}C{H_3}COOH$
The overall reaction can be shown as:
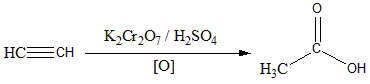
Hence the correct answer is Option (C)
Option (A) would be incorrect because Potassium Dichromate is a strong oxidising agent, and the reaction will proceed further until no further oxidation can take place. Hence it will form the most oxidised product, which in this case is the Carboxylic acid. If mild oxidising agents like PCC were used, then the reaction would stop at the formation of the first oxidised product; aldehyde.
Hence the correct answer is Option (C).
Note:
Acetylene and acetic acid are the common names known for these compounds. The IUPAC names of these compounds are Ethyne (acetylene) and Ethanoic Acid (acetic acid). The major use of acetic acid in households is in the form of Vinegar, which is the diluted form of it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




