
Identify the product of Cummene-Peroxide reaction among the following.
A.Phenol + Acetaldehyde
B.Phenol + Acetone
C.Ethanol + Acetaldehyde
D.Ethanol + Acetone
Answer
583.8k+ views
Hint:The given reaction is Cumene-Peroxide rearrangement. It is a very important method in organic chemistry used for the preparation of phenol. Cumene is known as isopropyl benzene. Cumene-hydroperoxide is used as a polymerization initiator and for the synthesis of acetone and phenol.
Complete step by step answer:
As we know the starting material is benzene hydroperoxide.
The first step is the formation of benzene peroxide from cumene
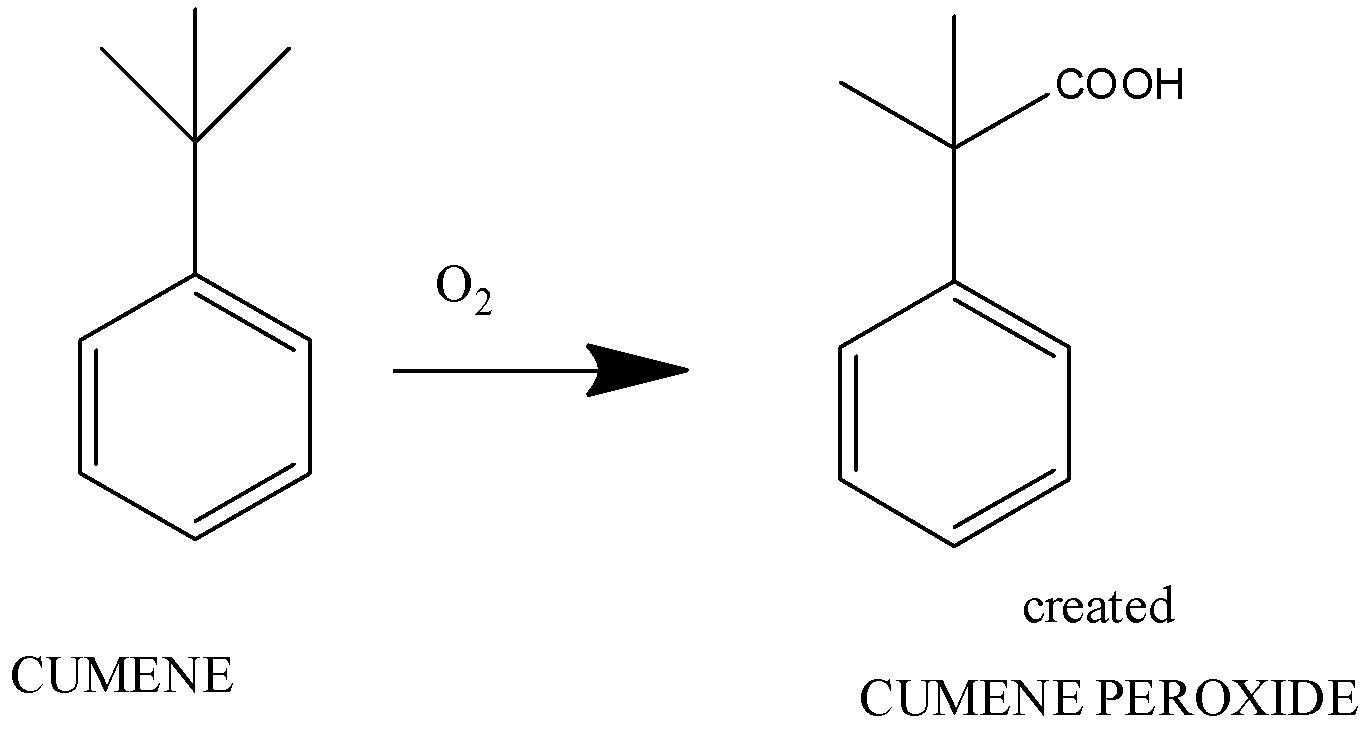
Here, cumene on adding oxygen forms cumene hydroperoxide.
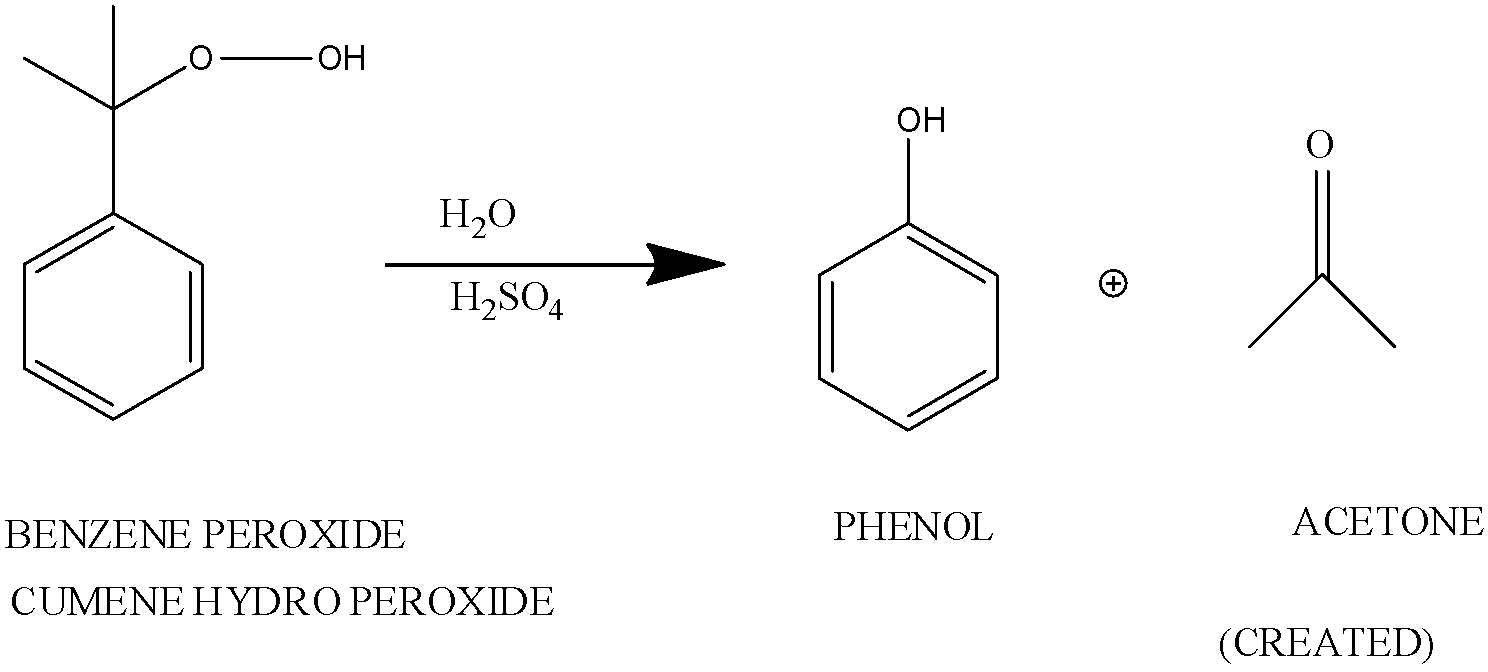
The tertiary hydroperoxide undergoes rearrangement in an acidic medium.
When cumene hydroperoxide is hydrolyzed in an acidic medium. This is known as Hock Rearrangement.
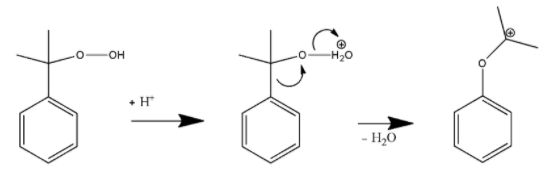
In the first step, the terminal hydroperoxy Oxygen atom will be protonated.
In the second step, the phenyl group then migrates from the benzyl carbon to the adjacent oxygen atom. It will further provide a resonance stabilized tertiary carbocation.
This is known as Baeyer- Villiger oxidation.
In this reaction, the carbocations are attacked by water and a proton is transferred from oxygen to the ether O. The ion then gives phenol and acetone.
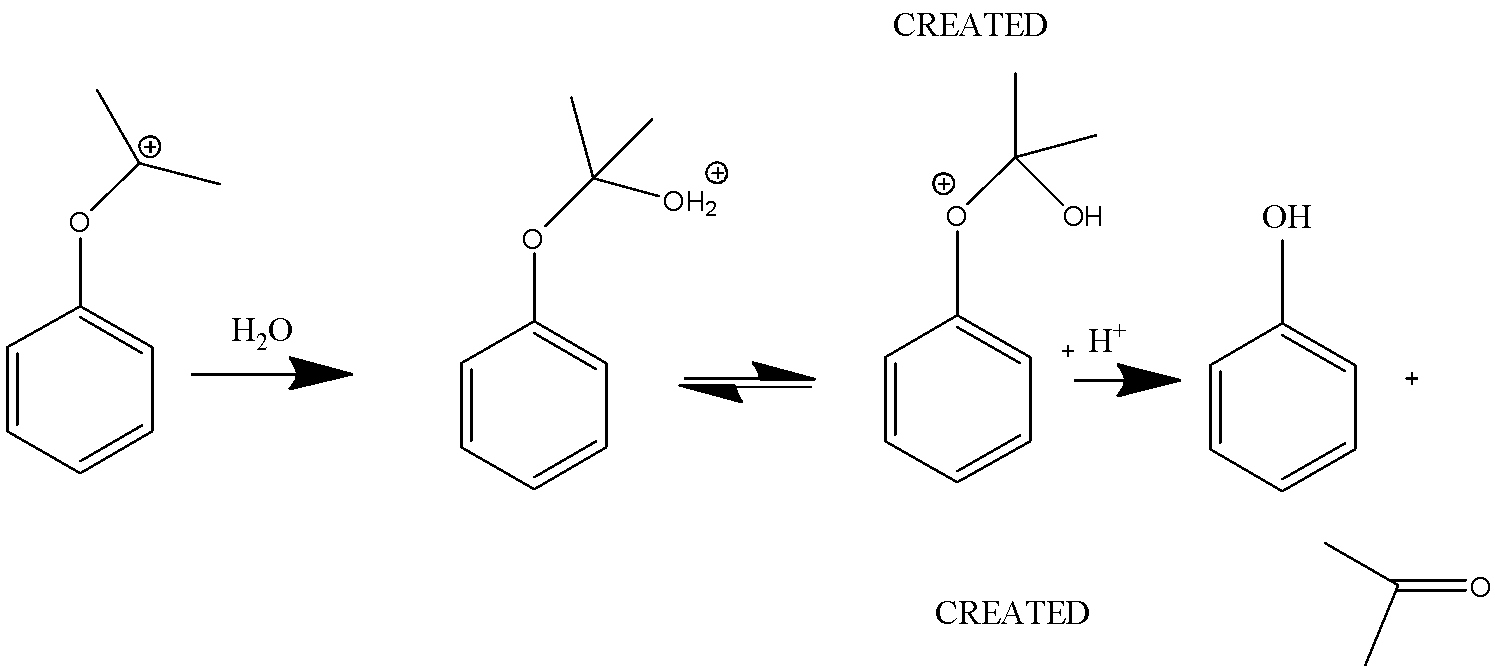
Therefore, the product of Cumene- hydroperoxide rearrangement reaction is acetone and phenol.
Hence, the correct option is (B).
Note: The Cumene-peroxide rearrangement or Hock rearrangement is used for the preparation of phenol. On decomposing cumene hydroperoxide, we get methyl styrene, acetophenone, and cumy alcohol. If the isopropyl benzene or the cumene is replaced with cyclohexyl benzene, it cleaves to give phenol and cyclohexanone. It is used as a precursor for the synthesis of nylon. 2-naphthol can by a method analogous to the Cumene process.
Complete step by step answer:
As we know the starting material is benzene hydroperoxide.
The first step is the formation of benzene peroxide from cumene

Here, cumene on adding oxygen forms cumene hydroperoxide.
The tertiary hydroperoxide undergoes rearrangement in an acidic medium.
When cumene hydroperoxide is hydrolyzed in an acidic medium. This is known as Hock Rearrangement.

In the first step, the terminal hydroperoxy Oxygen atom will be protonated.
In the second step, the phenyl group then migrates from the benzyl carbon to the adjacent oxygen atom. It will further provide a resonance stabilized tertiary carbocation.
This is known as Baeyer- Villiger oxidation.

In this reaction, the carbocations are attacked by water and a proton is transferred from oxygen to the ether O. The ion then gives phenol and acetone.

Therefore, the product of Cumene- hydroperoxide rearrangement reaction is acetone and phenol.
Hence, the correct option is (B).
Note: The Cumene-peroxide rearrangement or Hock rearrangement is used for the preparation of phenol. On decomposing cumene hydroperoxide, we get methyl styrene, acetophenone, and cumy alcohol. If the isopropyl benzene or the cumene is replaced with cyclohexyl benzene, it cleaves to give phenol and cyclohexanone. It is used as a precursor for the synthesis of nylon. 2-naphthol can by a method analogous to the Cumene process.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




