
Identify the odd one out of the following:
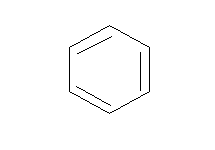
A.Benzene (\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\])
B.Cyclobutane (${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$)

C.Cyclopentane (${{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}$)
D.Cyclohexane (${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{10}}}}$)




Answer
584.1k+ views
Hint: Organic cyclic compounds are divided into three categories:
-Aromatic – are cyclic, conjugated and have (4n+2)pi electrons
-Anti-aromatic – are cyclic, conjugated and have (4n) pi electrons
-Non-Aromatic – are those compounds which do not fall under the aromatic or antiaromatic category.
Complete step by step answer:
Benzene: Benzene is a cyclic compound having conjugation (alternative single and double bond). There are 6 pi electrons in the benzene, thus following the (4n+2) pi-electron rule where n= 1.
This means benzene is an aromatic compound.
Cyclobutane: Cyclobutane is a cyclic compound but it does not have any conjugation and no pi electrons. Therefore it has neither (4n+2) pi electrons nor 4n pi electrons. Thus it falls under the category of Non-aromatic compounds
Cyclopentane: Cyclopentane is a cyclic molecule but has no conjugation and pi electrons are also absent. Since it does not satisfy the conditions of either aromatic or ant-aromatic, it is a non-aromatic compound.
Cyclohexane: Cyclohexane is again a cyclic compound but like cyclobutane and cyclopentane it does not satisfy the condition of either aromatic or antiaromatic and hence comes under non-aromatic compounds.
The above points indicated that among all the given four compounds, benzene is the only one which is aromatic and the rest of the three are non-aromatic compounds.
Hence, the correct answer is A.
Note:
Since we have talked about conjugation as a necessary condition for aromatic and antiaromatic compounds, it will be good to know what conjugation is. A conjugated system is a system of connected p-orbitals with delocalised electrons in a molecule. Conjugation is responsible for extra stability of the molecule.
-Aromatic – are cyclic, conjugated and have (4n+2)pi electrons
-Anti-aromatic – are cyclic, conjugated and have (4n) pi electrons
-Non-Aromatic – are those compounds which do not fall under the aromatic or antiaromatic category.
Complete step by step answer:
Benzene: Benzene is a cyclic compound having conjugation (alternative single and double bond). There are 6 pi electrons in the benzene, thus following the (4n+2) pi-electron rule where n= 1.
This means benzene is an aromatic compound.
Cyclobutane: Cyclobutane is a cyclic compound but it does not have any conjugation and no pi electrons. Therefore it has neither (4n+2) pi electrons nor 4n pi electrons. Thus it falls under the category of Non-aromatic compounds
Cyclopentane: Cyclopentane is a cyclic molecule but has no conjugation and pi electrons are also absent. Since it does not satisfy the conditions of either aromatic or ant-aromatic, it is a non-aromatic compound.
Cyclohexane: Cyclohexane is again a cyclic compound but like cyclobutane and cyclopentane it does not satisfy the condition of either aromatic or antiaromatic and hence comes under non-aromatic compounds.
The above points indicated that among all the given four compounds, benzene is the only one which is aromatic and the rest of the three are non-aromatic compounds.
Hence, the correct answer is A.
Note:
Since we have talked about conjugation as a necessary condition for aromatic and antiaromatic compounds, it will be good to know what conjugation is. A conjugated system is a system of connected p-orbitals with delocalised electrons in a molecule. Conjugation is responsible for extra stability of the molecule.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




