
Identify the odd one out from the following.
A. \({{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}\) Benzene
B. \({{\rm{C}}_{\rm{4}}}{{\rm{H}}_{\rm{8}}}\) Cyclobutane
C. \({{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}\) Cyclopentane
D. \({{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}\) Cyclohexane




Answer
585.9k+ views
Hint: We know that, if a compound is planar, cyclic, conjugated and follows Huckel’s rule of aromaticity, that is, 4n+2 pi electrons, then the compound is aromatic. To identify the odd one, we have to check the aromatic character of all the compounds.
Complete step by step answer: Let’s discuss the aromatic character of all the compounds given in the options.
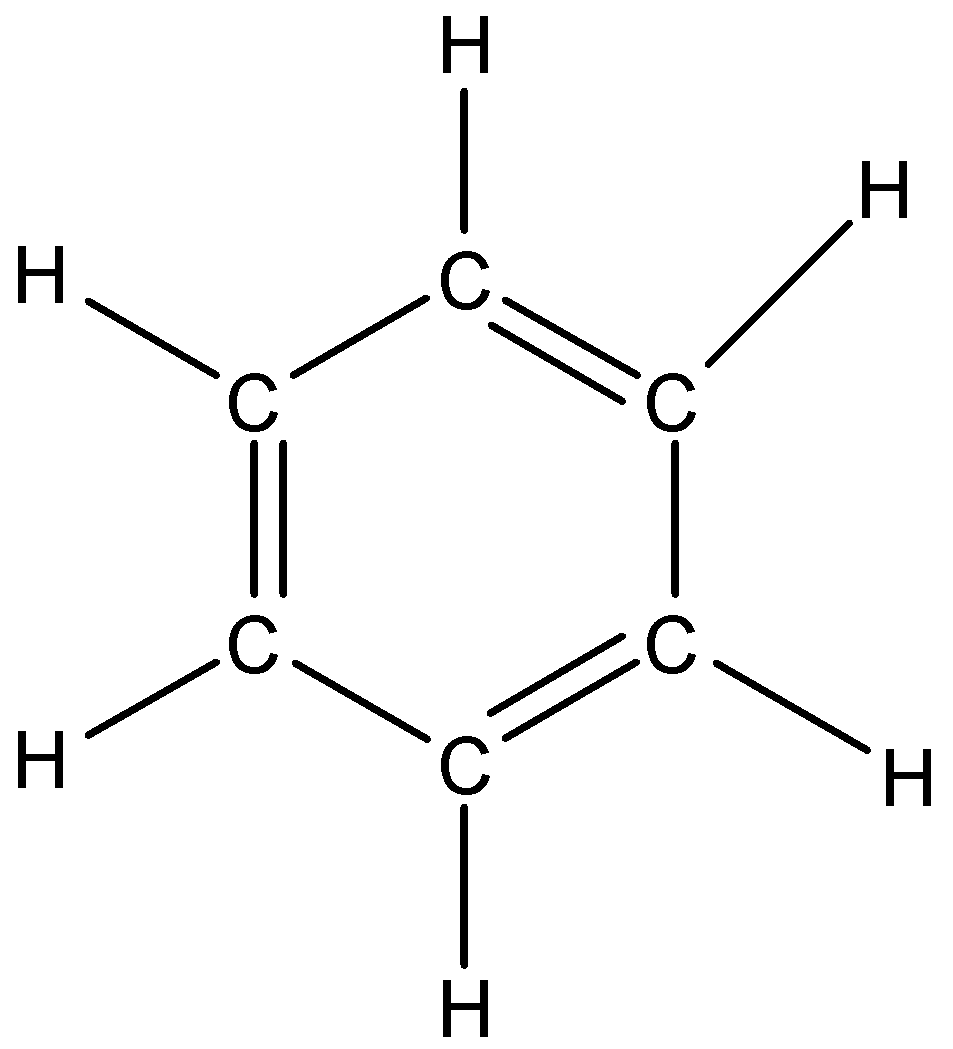
Option A is benzene \(\left( {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}} \right)\). We know the structure of benzene that is,
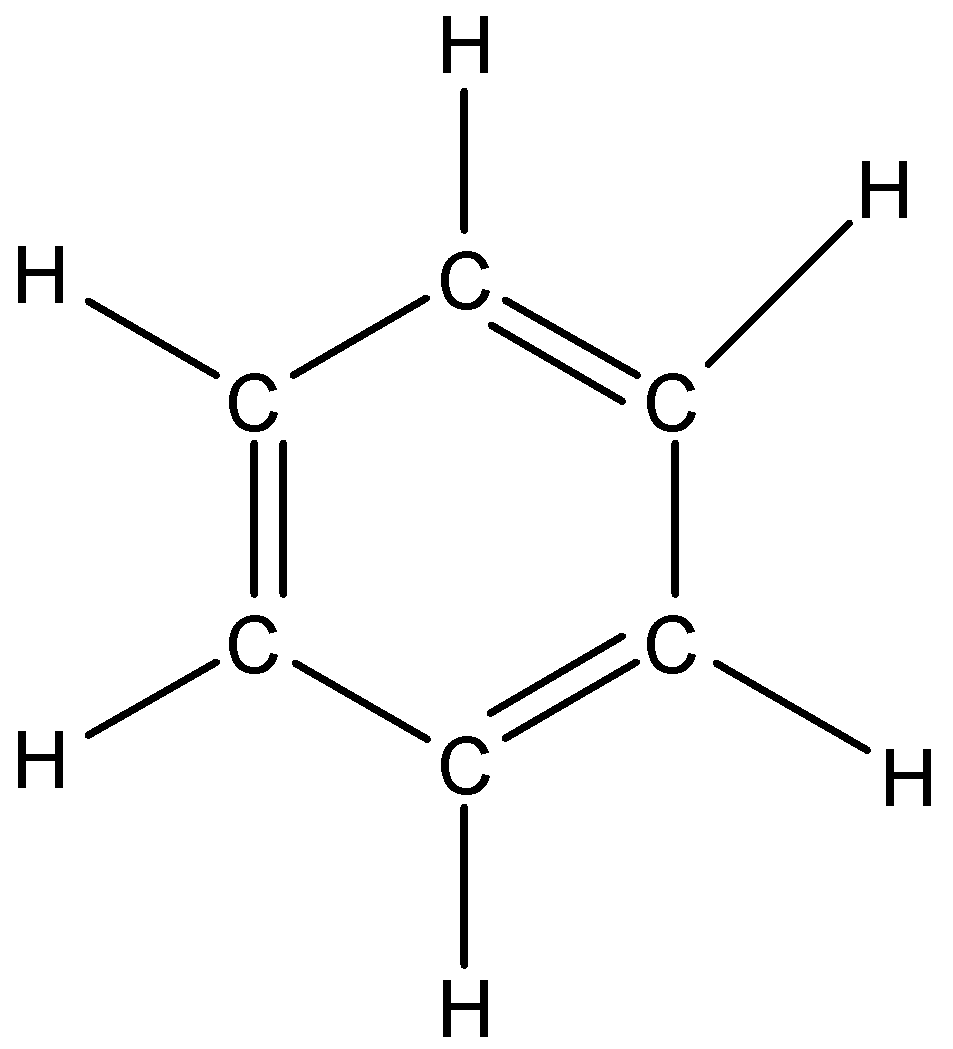
The number of pi electrons in the compound is 6. So, it follows the Huckel’s rule of aromaticity \(\left( {4n + 2} \right)\) for n=2. Therefore, benzene is an aromatic compound.
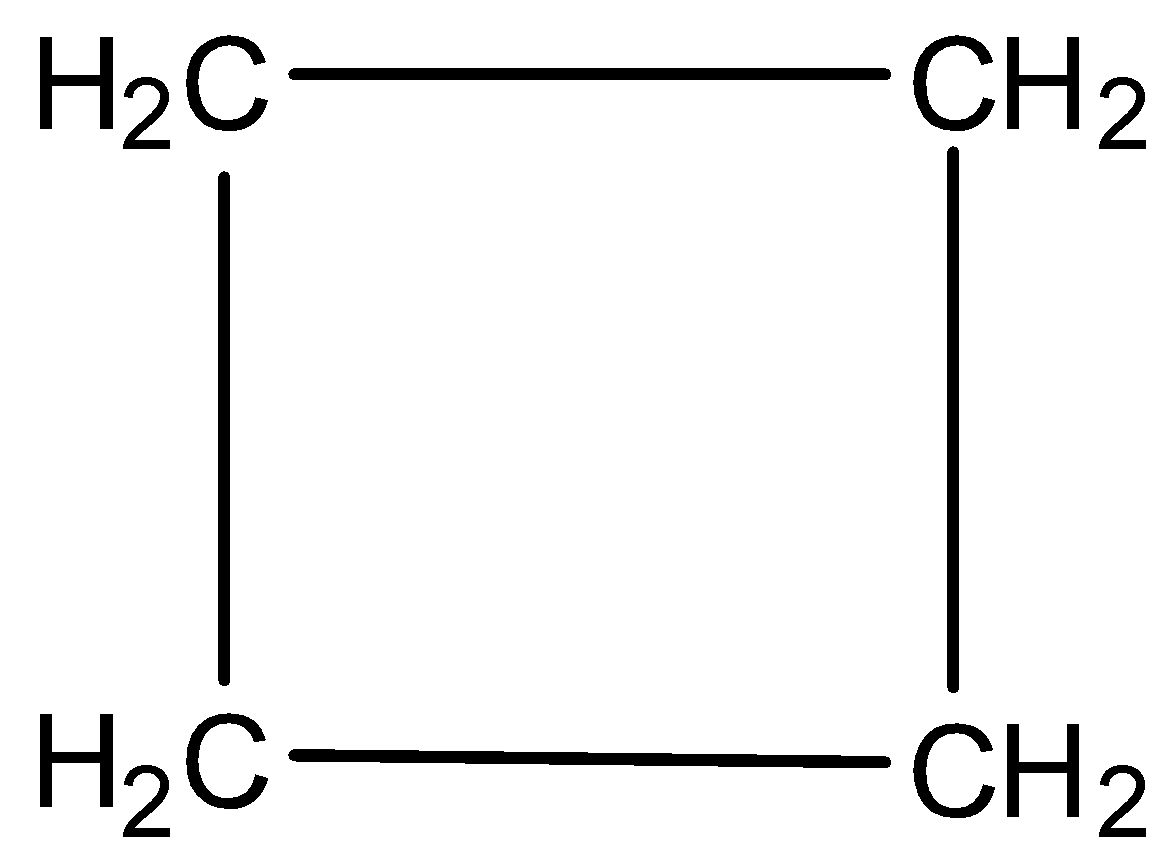
Now, we will discuss the aromatic character of cyclobutane. The structure of cyclobutane is
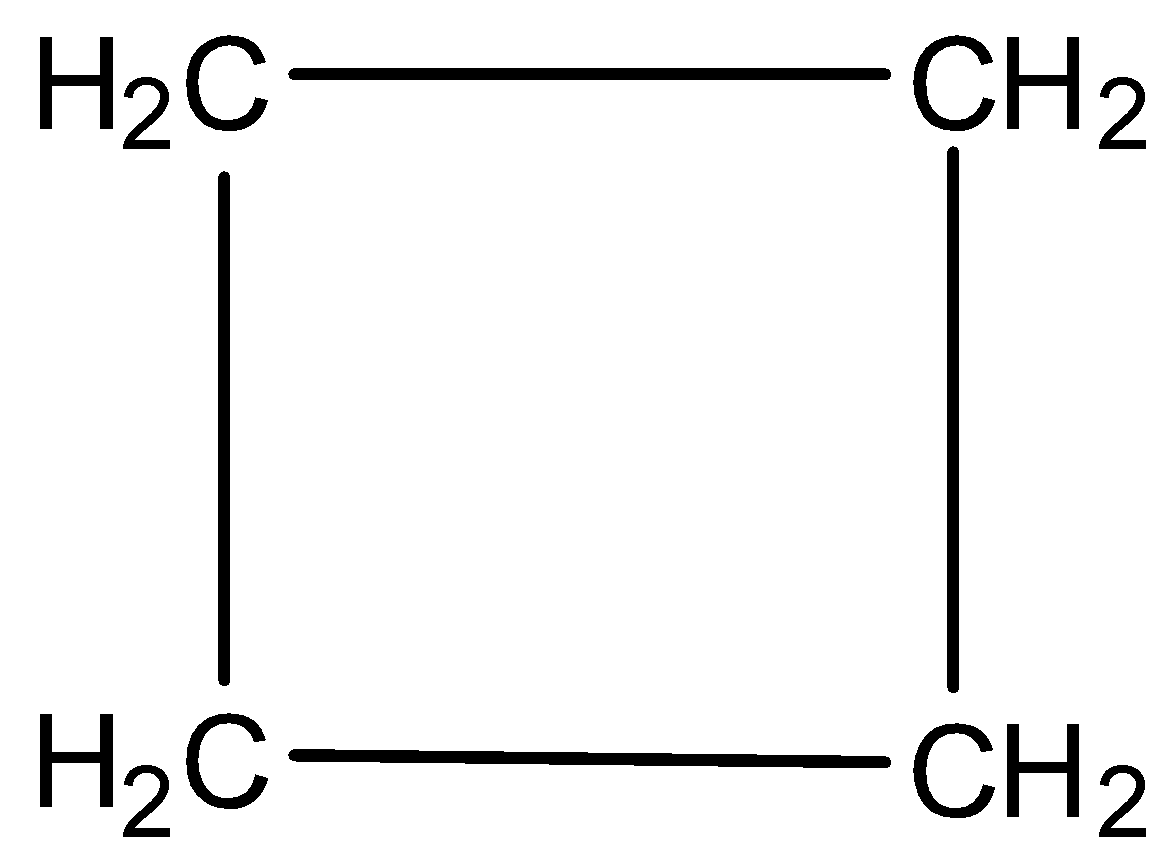
So, from the figure it is clear that no double bond exists, that means, no pi electrons. No pi electrons indicate that the compound is non aromatic.
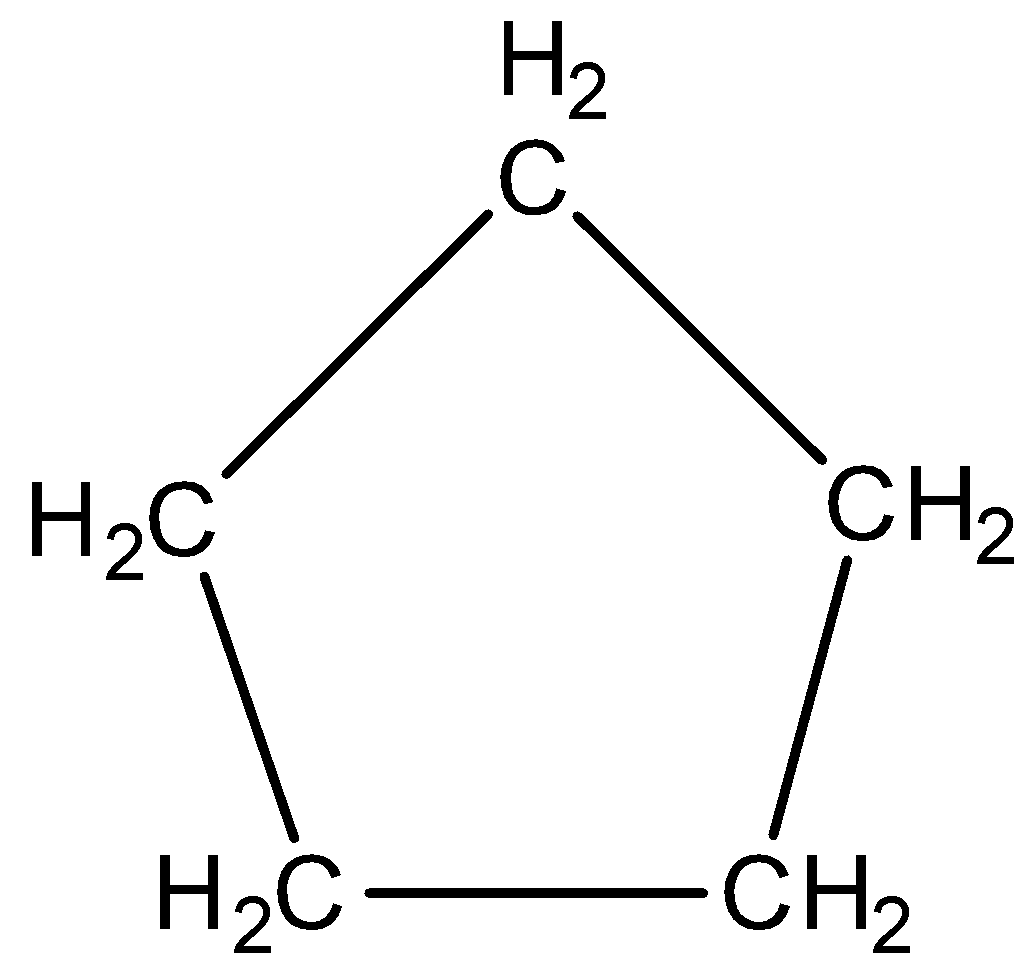
Now, we will discuss the aromatic character of cyclopentane. The structure of cyclopentane is,
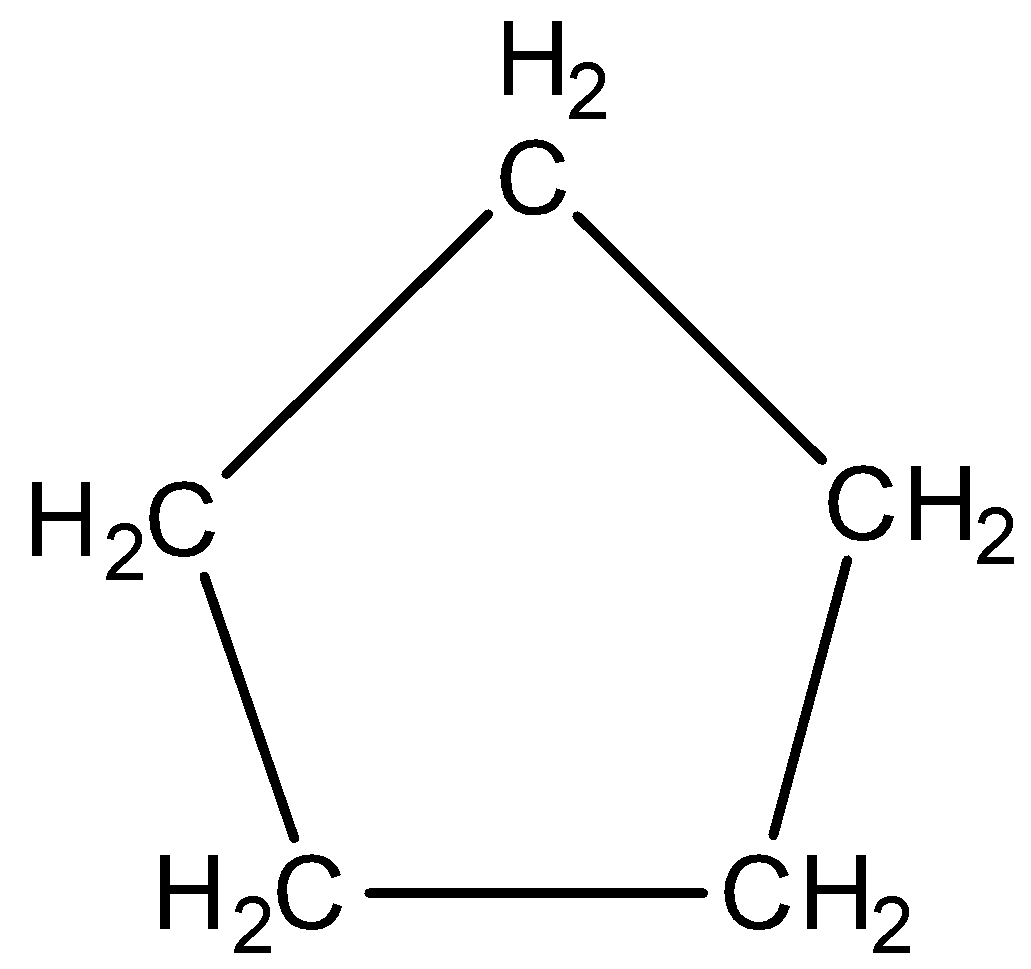
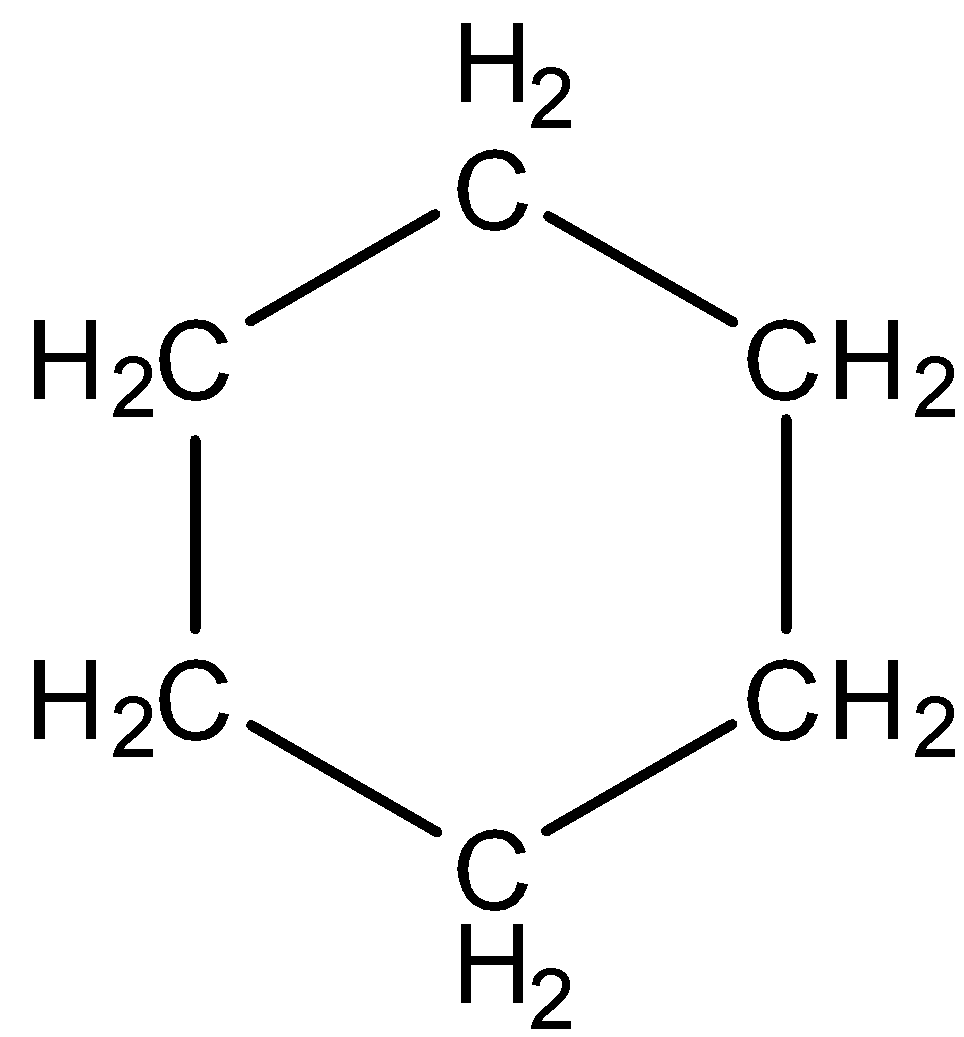
In cyclohexane no double bonds are present. This indicates the absence of pi electrons in the compound. Therefore, cyclohexane is also a non-aromatic compound.
So, we get to know that benzene is aromatic and all other compounds are non-aromatic. Therefore, the correct option is A, that is, benzene.
So, the correct answer is “Option A”.
Note: There are two terms antiaromatic and non-aromatic. If a compound is planar, cyclic, conjugated and follows 4n pi electrons, then the compound is antiaromatic. Non aromatic compounds are those compounds that do not belong to both aromatic compounds and antiaromatic compounds.
Complete step by step answer: Let’s discuss the aromatic character of all the compounds given in the options.
Option A is benzene \(\left( {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}} \right)\). We know the structure of benzene that is,

The number of pi electrons in the compound is 6. So, it follows the Huckel’s rule of aromaticity \(\left( {4n + 2} \right)\) for n=2. Therefore, benzene is an aromatic compound.
Now, we will discuss the aromatic character of cyclobutane. The structure of cyclobutane is

So, from the figure it is clear that no double bond exists, that means, no pi electrons. No pi electrons indicate that the compound is non aromatic.
Now, we will discuss the aromatic character of cyclopentane. The structure of cyclopentane is,

In cyclohexane no double bonds are present. This indicates the absence of pi electrons in the compound. Therefore, cyclohexane is also a non-aromatic compound.
So, we get to know that benzene is aromatic and all other compounds are non-aromatic. Therefore, the correct option is A, that is, benzene.
So, the correct answer is “Option A”.
Note: There are two terms antiaromatic and non-aromatic. If a compound is planar, cyclic, conjugated and follows 4n pi electrons, then the compound is antiaromatic. Non aromatic compounds are those compounds that do not belong to both aromatic compounds and antiaromatic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




