
Identify the monosaccharide containing only one asymmetrical carbon.
(A) Ribulose
(B) Ribose
(C) Erythrose
(D) Glyceraldehyde
Answer
589.2k+ views
Hint: Asymmetrical carbon is a carbon atom which is linked to four different substituents. Such carbon containing compounds are known as optically active compounds and that carbon atom is called a chiral carbon.
Monosaccharides are those carbohydrate molecules which cannot be hydrolyzed to give simpler sugars.
Complete step by step answer:
Let’s look at the solution of the given question.
To answer this question we will look for the carbon atom which is attached to four different groups or atoms in the given compounds.
(A) A ribulose is a five carbon monosaccharide containing a ketone functional group. Its structure can be made as:
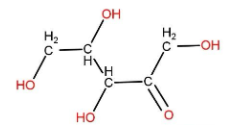
None of the carbon present is attached to four different atoms or groups. So, it doesn’t contain asymmetric carbon.
(B) A ribose is a five carbon sugar. Its d-ribose form is a component of RNA. It is found in cyclic structure but can be represented in open structure also. Its open structure can be made as:
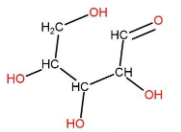
In this also none of the carbon atoms is attached to four different atoms or groups. So, it is an incorrect choice.
(C) Erythrose is tetrose saccharide. It is a four carbon sugar. It contains an aldehyde group. Its structure can be made as:
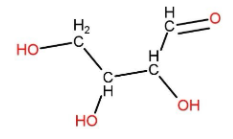
In this also none of the carbon atoms contains a carbon atom attached to four different atoms or groups.
(D) Glyceraldehyde is a triose monosaccharide. It is a three carbon sugar containing an aldehyde group. Its structure can be made as:
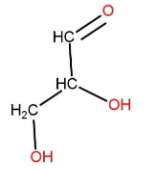
In this sugar the second carbon is attached to four different atoms and groups. So, it contains the asymmetrical carbon.
Hence, the answer is option (D).
Note: A carbon which is attached to four different substituents is called an asymmetric carbon. It is also called chirality of the molecule. While looking for chiral carbon we do not consider double bonded and triple bonded carbon atoms as the number of bonds formed in these cases is less than four.
Monosaccharides are those carbohydrate molecules which cannot be hydrolyzed to give simpler sugars.
Complete step by step answer:
Let’s look at the solution of the given question.
To answer this question we will look for the carbon atom which is attached to four different groups or atoms in the given compounds.
(A) A ribulose is a five carbon monosaccharide containing a ketone functional group. Its structure can be made as:

None of the carbon present is attached to four different atoms or groups. So, it doesn’t contain asymmetric carbon.
(B) A ribose is a five carbon sugar. Its d-ribose form is a component of RNA. It is found in cyclic structure but can be represented in open structure also. Its open structure can be made as:

In this also none of the carbon atoms is attached to four different atoms or groups. So, it is an incorrect choice.
(C) Erythrose is tetrose saccharide. It is a four carbon sugar. It contains an aldehyde group. Its structure can be made as:

In this also none of the carbon atoms contains a carbon atom attached to four different atoms or groups.
(D) Glyceraldehyde is a triose monosaccharide. It is a three carbon sugar containing an aldehyde group. Its structure can be made as:

In this sugar the second carbon is attached to four different atoms and groups. So, it contains the asymmetrical carbon.
Hence, the answer is option (D).
Note: A carbon which is attached to four different substituents is called an asymmetric carbon. It is also called chirality of the molecule. While looking for chiral carbon we do not consider double bonded and triple bonded carbon atoms as the number of bonds formed in these cases is less than four.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




