
Identify the \[M{g^{2 + }}\] ion, where n and p represent the number of neutrons and protons respectively.
A.
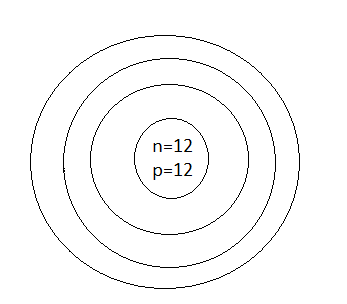
B.
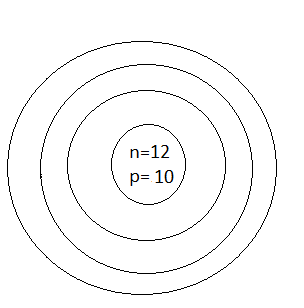
C.
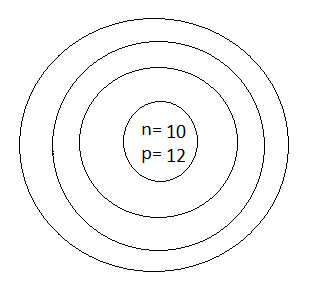
D.
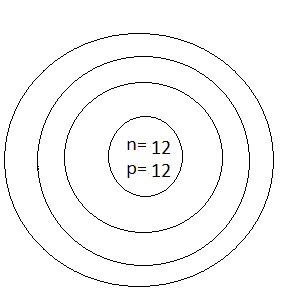




Answer
477.9k+ views
Hint: An atom consists of subatomic particles like electrons, protons, and neutrons. In every neutral atom, the number of protons is equal to the number of electrons. if it is an ion, the electrons will change but not protons. The number of neutrons can be determined by the subtraction of an atomic number from a mass number.
Complete answer:
A chemical element is the purest form of an atom and cannot be broken into simple units by any chemical action. An atom is the smallest particle that cannot be visible to a human eye.
When a neutral atom loses or gains electrons, it becomes an ion.
Given ion is \[M{g^{2 + }}\] , magnesium is the element with atomic number \[12\] , which is equal to the number of protons. So, the number of electrons will also be \[12\] .
The atomic number is nothing but the number of protons, whereas the atomic mass of magnesium is \[24\] atomic mass units. Thus, the number of neutrons are \[12\] in magnesium ions or \[M{g^{2 + }}\].
Thus, the number of protons which was represented by p is \[12\] and the number of neutrons which was represented by n is also \[12\]
Thus, option D is the correct one.
Note:
The atomic number represented by \[Z\] is equal to the number of protons. As an atom must be stable, it should have an equal number of protons and electrons. Thus, a neutral atom has an equal number of protons and electrons, which is different in the case of ions.
Complete answer:
A chemical element is the purest form of an atom and cannot be broken into simple units by any chemical action. An atom is the smallest particle that cannot be visible to a human eye.
When a neutral atom loses or gains electrons, it becomes an ion.
Given ion is \[M{g^{2 + }}\] , magnesium is the element with atomic number \[12\] , which is equal to the number of protons. So, the number of electrons will also be \[12\] .
The atomic number is nothing but the number of protons, whereas the atomic mass of magnesium is \[24\] atomic mass units. Thus, the number of neutrons are \[12\] in magnesium ions or \[M{g^{2 + }}\].
Thus, the number of protons which was represented by p is \[12\] and the number of neutrons which was represented by n is also \[12\]
Thus, option D is the correct one.
Note:
The atomic number represented by \[Z\] is equal to the number of protons. As an atom must be stable, it should have an equal number of protons and electrons. Thus, a neutral atom has an equal number of protons and electrons, which is different in the case of ions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




