
How do you identify the compound for IR Spectrum for the molecular formula \[{C_5}{H_8}O\]?
Answer
510.3k+ views
Hint: We have to know that the IR range starts from $800$ to $4000c{m^{ - 1}}$. We can observe and measure this “singing” of bonds by applying IR radiation to a sample and measuring the frequencies at which the radiation is absorbed. The result is a technique known as Infrared Spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule.
Complete answer:
IR-frequency light is passed through a compound. The amount and frequencies of the light absorbed is related to the functional groups and structure of the compound. This helps us to identify the compound. All “spectroscopy” methods use light wavelengths from infrared to UV.
The compound with molecular formula \[{C_5}{H_8}O\], contains oxygen atoms. The probable compound must have a ketonic group in it as it has one oxygen atom. Also we can look for aldehydes and alcohols as they also contain one oxygen atom.
So when we derive the structure for this compound we get: This can be the most probable compound having molecular formula \[{C_5}{H_8}O\].
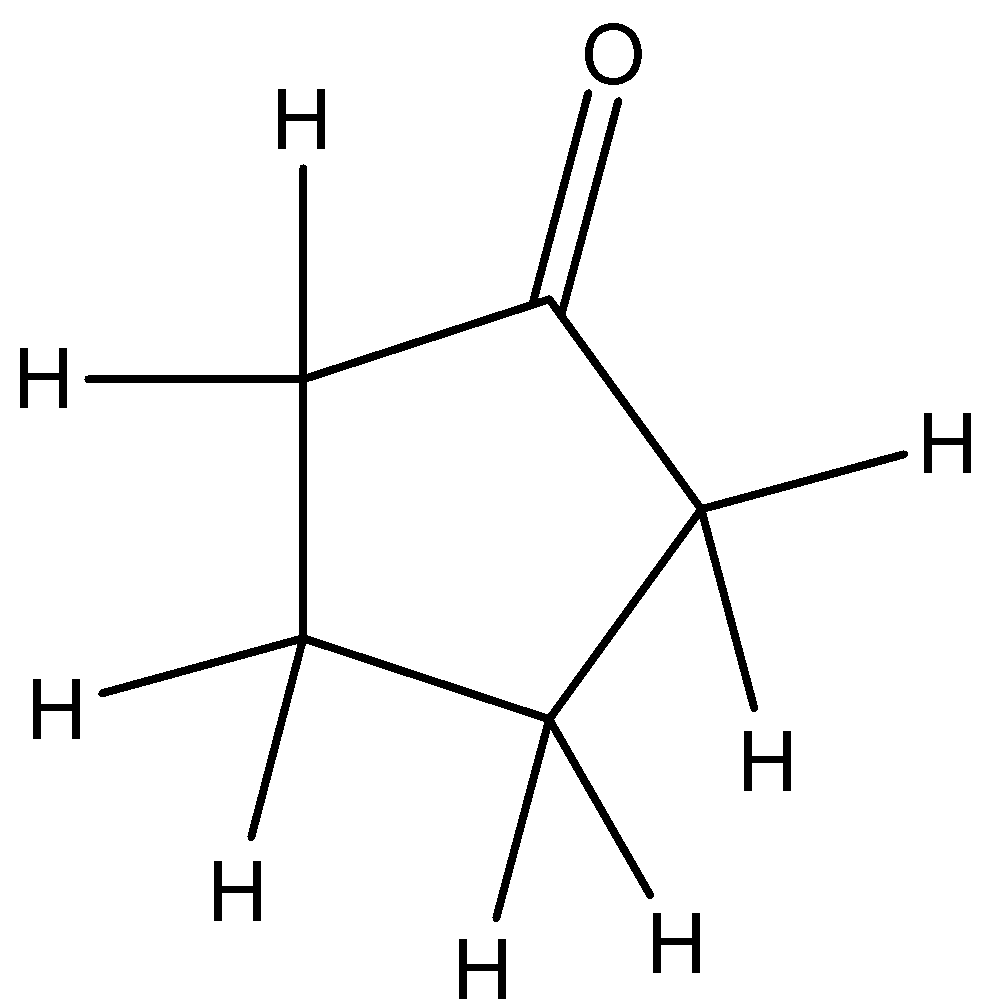
The Infra-Red (IR) spectrum of acyclic ketones shows a strong $C = O$ stretching absorption at \[1715c{m^{ - 1}}\].
The other important vibrational peak of cyclopentanone molecule is of carbon-hydrogen bond, which is at \[3000cm - 1\]. At \[1500c{m^{ - 1}}\], saturated carbon- carbon bending mode vibrations occur. In the fingerprint region, carbon hydrogen wagging mode vibrations occur.
Note:
As we move from acyclic to cyclic ketones the value of this absorption rises as the ring-size decreases from $6$ to $3$ .Cyclic ketones don't terminate with methyl groups but only show signals and splitting pattern for methylene groups which make up their ring structure.
Complete answer:
IR-frequency light is passed through a compound. The amount and frequencies of the light absorbed is related to the functional groups and structure of the compound. This helps us to identify the compound. All “spectroscopy” methods use light wavelengths from infrared to UV.
The compound with molecular formula \[{C_5}{H_8}O\], contains oxygen atoms. The probable compound must have a ketonic group in it as it has one oxygen atom. Also we can look for aldehydes and alcohols as they also contain one oxygen atom.
So when we derive the structure for this compound we get: This can be the most probable compound having molecular formula \[{C_5}{H_8}O\].

The Infra-Red (IR) spectrum of acyclic ketones shows a strong $C = O$ stretching absorption at \[1715c{m^{ - 1}}\].
The other important vibrational peak of cyclopentanone molecule is of carbon-hydrogen bond, which is at \[3000cm - 1\]. At \[1500c{m^{ - 1}}\], saturated carbon- carbon bending mode vibrations occur. In the fingerprint region, carbon hydrogen wagging mode vibrations occur.
Note:
As we move from acyclic to cyclic ketones the value of this absorption rises as the ring-size decreases from $6$ to $3$ .Cyclic ketones don't terminate with methyl groups but only show signals and splitting pattern for methylene groups which make up their ring structure.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




