
Identify the compound C.
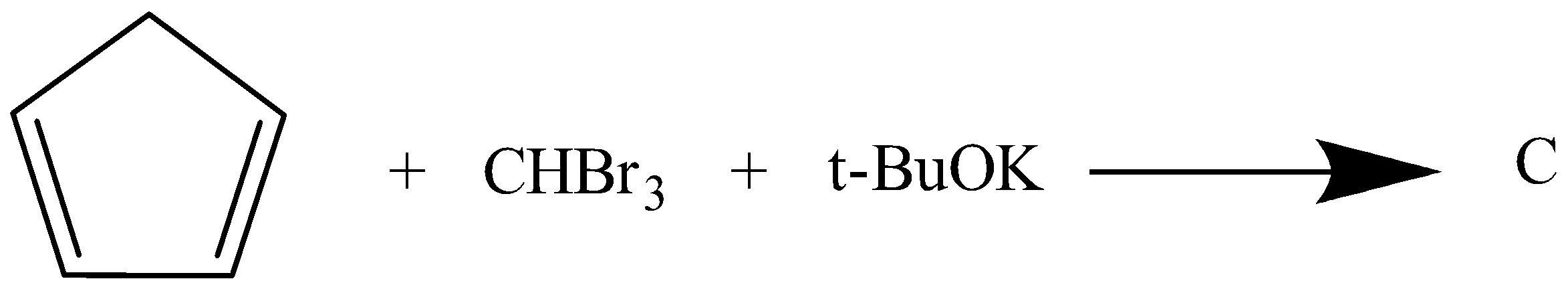
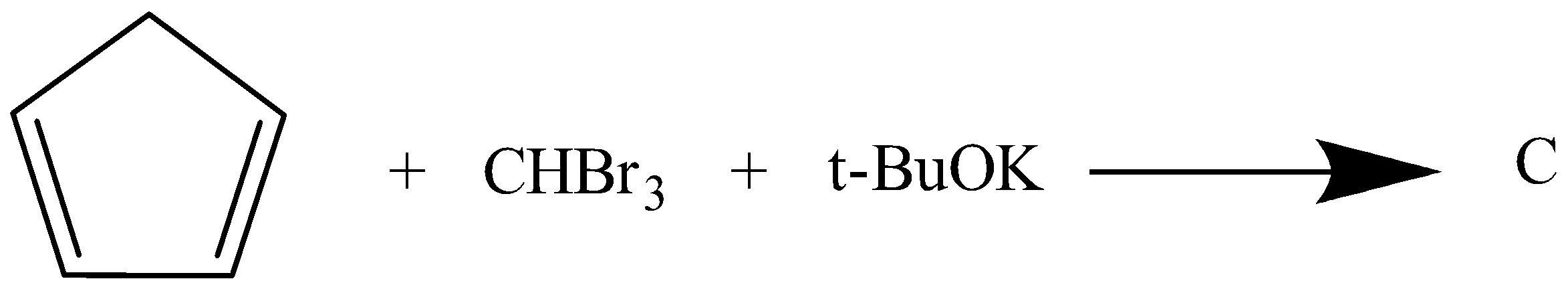
Answer
537.6k+ views
Hint: The $ t - BuOK $ is the tert- butoxide which is the conjugate base of tert- butanol. The potassium tert- butoxide helps in catalyzing the reaction of hydrosilanes and heterocyclic compounds which helps in formation of silyl derivatives. The tert- butoxide is a strong base because of the inductive effect and the presence of electron donating methyl groups.
Complete step by step solution:
The methyl groups in tert- butoxide reduces the polarity of hydroxide bond in t- butyl alcohol which makes it a weaker acid. And a strong conjugate base. The reason is salvation also because it is difficult for tert- butoxide to dissociate into ions so that the compound gets destabilised into ethoxide. It is a weak nucleophile because of the presence of steric hindrance. So when the above organic compound reacts with bromoform and tert- butoxide the formation of the following product occurs.
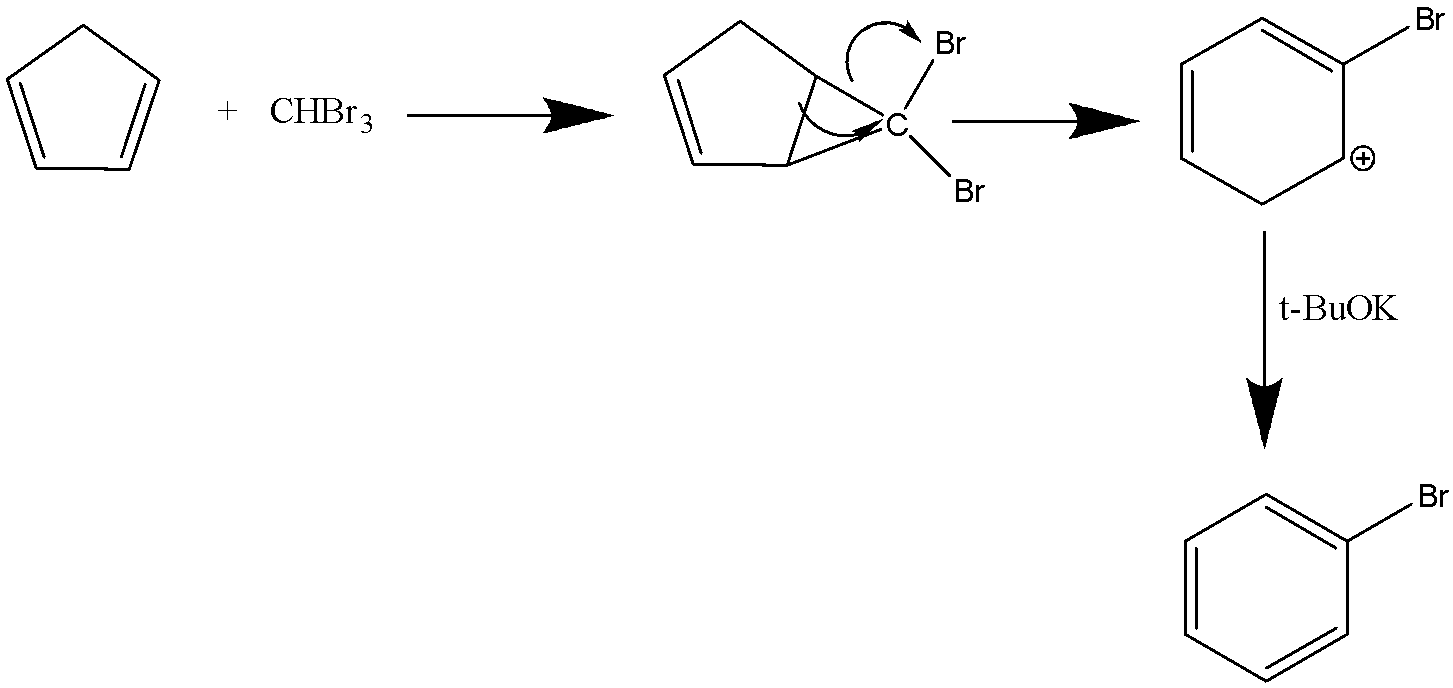
The product formed after the reaction is bromobenzene .In the above reaction Haloform reaction has occurred. In this mechanism the disproportion of the halogen with presence of hydroxide ion occurs. The reaction is of the nucleophilic substitution reaction. The tert- butoxide exists as a tetrameric cubane type cluster. It is a colourless solid which is widely used in the formation of organic compounds.
Hence, the compound C is bromobenzene ( $ {C_6}{H_5}Br $ ).
Note:
The tert butoxide is a non nucleophilic strong base. Due to its steric hindrance by the methyl groups it can participate in nucleophilic reaction. The compound formed in the reaction is complex in nature which affects the reactivity of reagent. Schlosser’s base is a mixture of the alkoxide and alkyl lithium compound, and is a related but stronger base.
Complete step by step solution:
The methyl groups in tert- butoxide reduces the polarity of hydroxide bond in t- butyl alcohol which makes it a weaker acid. And a strong conjugate base. The reason is salvation also because it is difficult for tert- butoxide to dissociate into ions so that the compound gets destabilised into ethoxide. It is a weak nucleophile because of the presence of steric hindrance. So when the above organic compound reacts with bromoform and tert- butoxide the formation of the following product occurs.

The product formed after the reaction is bromobenzene .In the above reaction Haloform reaction has occurred. In this mechanism the disproportion of the halogen with presence of hydroxide ion occurs. The reaction is of the nucleophilic substitution reaction. The tert- butoxide exists as a tetrameric cubane type cluster. It is a colourless solid which is widely used in the formation of organic compounds.
Hence, the compound C is bromobenzene ( $ {C_6}{H_5}Br $ ).
Note:
The tert butoxide is a non nucleophilic strong base. Due to its steric hindrance by the methyl groups it can participate in nucleophilic reaction. The compound formed in the reaction is complex in nature which affects the reactivity of reagent. Schlosser’s base is a mixture of the alkoxide and alkyl lithium compound, and is a related but stronger base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




