
Identify the compound (A) in the reaction:
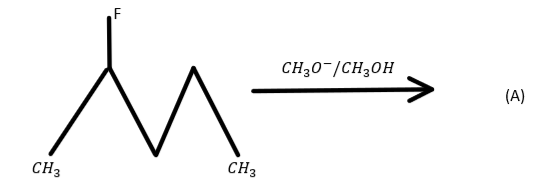
A.

B.

C.
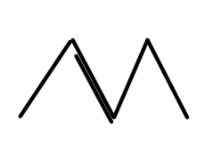
D.





Answer
573.3k+ views
Hint: We know that when haloalkanes react with $C{H_3}{O^ - }$ dissolved in ethanol then they form alkenes. And which alkene will form is decided by the number of hydrogen atoms at the adjacent carbon atoms.
Complete step by step solution:
Let us first talk about the term alkenes, alkanes and alkynes.
Alkanes have the general formula as ${C_n}{H_{(2n + 2)}}$, where $n$ is the number of carbon atoms in the hydrocarbon. Alkenes have the general formula as ${C_n}{H_{(2n)}}$, where $n$ is the number of carbon atoms in the hydrocarbon. And similarly alkynes have the general formula as ${C_n}{H_{(2n - 2)}}$, where $n$ is the number of carbon atoms in the hydrocarbon.
Halo-alkanes: They are defined as the alkanes in which one hydrogen atom is replaced by one halogen atom i.e. fluorine, chlorine, iodine, etc.
Now we know that when haloalkanes react with $C{H_3}{O^ - }$ dissolved in ethanol then they form alkenes. And which alkene will form is decided by the number of hydrogen atoms at the adjacent carbon atoms. And which alkene will form is decided by the number of hydrogen atoms at the adjacent carbon atoms. The alkene which will have the largest number of hydrogen atoms at the adjacent carbon atom will form in large quantity and other will also produce but in small quantity. So in the question the reaction of $2 - $fluoro pentane with $C{H_3}{O^ - }$ dissolved in ethanol is given. So the product formed will be ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = CHC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}$ because in this compounds number of hydrogen atoms attached to the adjacent carbon is high i.e. $5$.
In option A number of hydrogen atoms attached to the adjacent carbon is $3$.
In option B the number of hydrogen atoms attached to the adjacent carbon is $2$.
In option B the number of hydrogen atoms attached to the adjacent carbon is $5$. But in this number of carbon atoms are greater than the number of carbon atoms in the reactants. So it is wrong.
So, option C is correct.
Note: Isomerism: This is defined as the phenomenon in which more than one compounds have the same chemical formula but they have different chemical structures.
Structural isomerism: They are defined as the compounds which differ in the connectivity between the atoms that constitute the compound d.
Stereoisomerism: The compounds which have the same molecular formula but differ in the arrangements of atoms in three-dimensional structure.
Complete step by step solution:
Let us first talk about the term alkenes, alkanes and alkynes.
Alkanes have the general formula as ${C_n}{H_{(2n + 2)}}$, where $n$ is the number of carbon atoms in the hydrocarbon. Alkenes have the general formula as ${C_n}{H_{(2n)}}$, where $n$ is the number of carbon atoms in the hydrocarbon. And similarly alkynes have the general formula as ${C_n}{H_{(2n - 2)}}$, where $n$ is the number of carbon atoms in the hydrocarbon.
Halo-alkanes: They are defined as the alkanes in which one hydrogen atom is replaced by one halogen atom i.e. fluorine, chlorine, iodine, etc.
Now we know that when haloalkanes react with $C{H_3}{O^ - }$ dissolved in ethanol then they form alkenes. And which alkene will form is decided by the number of hydrogen atoms at the adjacent carbon atoms. And which alkene will form is decided by the number of hydrogen atoms at the adjacent carbon atoms. The alkene which will have the largest number of hydrogen atoms at the adjacent carbon atom will form in large quantity and other will also produce but in small quantity. So in the question the reaction of $2 - $fluoro pentane with $C{H_3}{O^ - }$ dissolved in ethanol is given. So the product formed will be ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = CHC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}$ because in this compounds number of hydrogen atoms attached to the adjacent carbon is high i.e. $5$.
In option A number of hydrogen atoms attached to the adjacent carbon is $3$.
In option B the number of hydrogen atoms attached to the adjacent carbon is $2$.
In option B the number of hydrogen atoms attached to the adjacent carbon is $5$. But in this number of carbon atoms are greater than the number of carbon atoms in the reactants. So it is wrong.
So, option C is correct.
Note: Isomerism: This is defined as the phenomenon in which more than one compounds have the same chemical formula but they have different chemical structures.
Structural isomerism: They are defined as the compounds which differ in the connectivity between the atoms that constitute the compound d.
Stereoisomerism: The compounds which have the same molecular formula but differ in the arrangements of atoms in three-dimensional structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




