
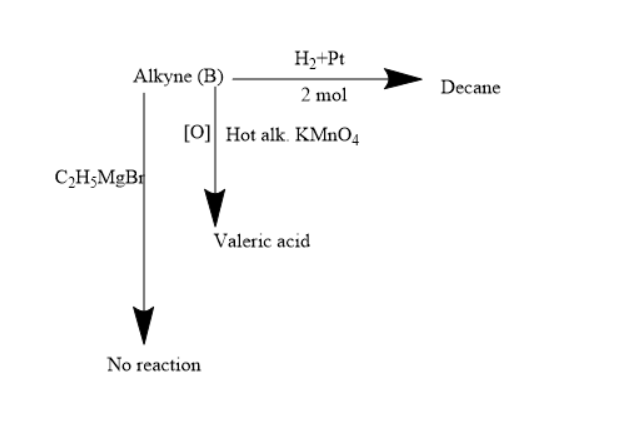
Identify the alkynes A, B, $ \left( {{C_{10}}{H_{18}}} \right) $ and $ \left( {{C_{10}}{H_{16}}} \right) $ which gives the following reactions. What is (B)?
$ A)Dec - 6 - yne $
$ B)Hex - 1 - yne $
$ C)Dec - 5 - yne $
$ D)Non - 5 - yne $

Answer
501.9k+ views
Hint :Alkynes are unsaturated hydrocarbons consisting of a triple bond and can be reduced to alkenes and alkanes by treating with hydrogen in presence of platinum. Thus, alkyne can be reduced to alkane. In presence of alkaline potassium permanganate cleaves the triple bond and forms acid. Grignard reagents do not react with alkynes.
Complete Step By Step Answer:
Given molecules are alkyne, alkyne when treated with hydrogen in the presence of platinum forms alkenes or alkanes. Given that decane is formed when alkyne is treated with hydrogen in presence of platinum. Thus, the alkyne will consist of $ 10 $ carbon atoms.
Given that alkyne treated with hot alkaline potassium permanganate, undergoes oxidation and forms valeric acid. Valeric acid is also known as pentatonic acid. Two moles of pentatonic acid will be formed in the given reaction. Thus, the position of the triple bond position will be at the $ {5^{th}} $ position of alkyne consisting of $ 10 $ carbon atoms.
The given alkynes do not react with Grignard reagents.
Thus, from all the above conditions and formation of products the alkyne (B) will be $ Dec - 5 - yne $ .
$ Dec - 6 - yne $ has the position of the alkyne functional group at $ {6^{th}} $ position. Thus, it is not the correct option.
In the given options $ Dec - 5 - yne $ is the C option.
Thus, option C is the correct one.
Note :
Potassium permanganate is an oxidizing agent and oxidises the alkynes to carboxylic acids. The cleavage of carbon -carbon bond takes place at the position of triple bond in alkynes. The $ Dec - 5 - yne $ is not a terminal alkyne, so it does not react with Grignard reagent.
Complete Step By Step Answer:
Given molecules are alkyne, alkyne when treated with hydrogen in the presence of platinum forms alkenes or alkanes. Given that decane is formed when alkyne is treated with hydrogen in presence of platinum. Thus, the alkyne will consist of $ 10 $ carbon atoms.
Given that alkyne treated with hot alkaline potassium permanganate, undergoes oxidation and forms valeric acid. Valeric acid is also known as pentatonic acid. Two moles of pentatonic acid will be formed in the given reaction. Thus, the position of the triple bond position will be at the $ {5^{th}} $ position of alkyne consisting of $ 10 $ carbon atoms.
The given alkynes do not react with Grignard reagents.
Thus, from all the above conditions and formation of products the alkyne (B) will be $ Dec - 5 - yne $ .
$ Dec - 6 - yne $ has the position of the alkyne functional group at $ {6^{th}} $ position. Thus, it is not the correct option.
In the given options $ Dec - 5 - yne $ is the C option.
Thus, option C is the correct one.
Note :
Potassium permanganate is an oxidizing agent and oxidises the alkynes to carboxylic acids. The cleavage of carbon -carbon bond takes place at the position of triple bond in alkynes. The $ Dec - 5 - yne $ is not a terminal alkyne, so it does not react with Grignard reagent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




