
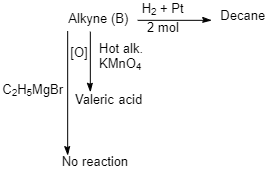
Identify the alkynes A, B $ ({C_{10}}{H_{16}}) $ and $ ({C_{10}}{H_{18}}) $ which gives the following reactions. What is (B)?

Answer
507k+ views
Hint: One can solve this question by hit and trial method but it takes more time. Decane is an alkane hydrocarbon with $ 10 $ carbon and $ 22 $ hydrogen with a single bond between its carbon atoms. It has $ 75 $ structural isomers and all the isomers exhibit similar properties. Decane is a nonpolar solvent and a component of petrol and kerosene.
Complete answer:
According to the question, the given unknown alkyne does not react with Grignard’s reagent, terminal alkyne contains acidic hydrogen so only terminal alkyne reacts with Grignard’s reagent.
So, when Dec-5-yne reacts with hydrogen in the presence of Pt, Decane is produced.
$ Dec - 5 - yne\xrightarrow{{{H_2} + Pt}}Decane $
and when the alkyne $ (Dec-5-yne) $ undergoes oxidation in the presence of hot alkaline $ KMn{O_4} $ produces Valeric acid $ C{H_3}{(C{H_2})_3}COOH $ .
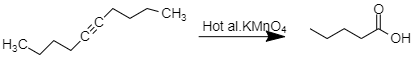
But when Dec-6-yne undergoes oxidation in the presence of hot alkaline $ $ $ KMn{O_4} $ product formed is other than pentanoic acid.
Therefore the correct answer is option C.
Additional Information:
Lindlar catalyst is a heterogeneous catalyst which comprises palladium deposited on barium sulphate or calcium carbonate poisoned by quinoline. It should be noted that the hydrogenation can be controlled at the alkene stage only. This is done by using a Lindlar’s catalyst. It allows the hydrogenation of alkynes only to the alkene stage.
Note:
Alkaline potassium permanganate is an oxidizing agent. Potassium permanganate is a purplish-black, crystalline salt. It is not only an oxidizing agent, it also has disinfectant, deodorising and astringent properties too. If potassium permanganate solution is acidified with dilute strong acid then the purple colour solution becomes colourless, while if the potassium permanganate solution is made alkaline then the purple colour solution will first become dark green in colour and then produce a dark brown precipitate.
Complete answer:
According to the question, the given unknown alkyne does not react with Grignard’s reagent, terminal alkyne contains acidic hydrogen so only terminal alkyne reacts with Grignard’s reagent.
So, when Dec-5-yne reacts with hydrogen in the presence of Pt, Decane is produced.
$ Dec - 5 - yne\xrightarrow{{{H_2} + Pt}}Decane $
and when the alkyne $ (Dec-5-yne) $ undergoes oxidation in the presence of hot alkaline $ KMn{O_4} $ produces Valeric acid $ C{H_3}{(C{H_2})_3}COOH $ .
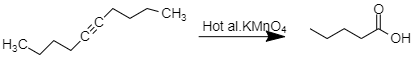
But when Dec-6-yne undergoes oxidation in the presence of hot alkaline $ $ $ KMn{O_4} $ product formed is other than pentanoic acid.
Therefore the correct answer is option C.
Additional Information:
Lindlar catalyst is a heterogeneous catalyst which comprises palladium deposited on barium sulphate or calcium carbonate poisoned by quinoline. It should be noted that the hydrogenation can be controlled at the alkene stage only. This is done by using a Lindlar’s catalyst. It allows the hydrogenation of alkynes only to the alkene stage.
Note:
Alkaline potassium permanganate is an oxidizing agent. Potassium permanganate is a purplish-black, crystalline salt. It is not only an oxidizing agent, it also has disinfectant, deodorising and astringent properties too. If potassium permanganate solution is acidified with dilute strong acid then the purple colour solution becomes colourless, while if the potassium permanganate solution is made alkaline then the purple colour solution will first become dark green in colour and then produce a dark brown precipitate.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




