
Identify Pyridinium chlorochromate from the following:
(A)
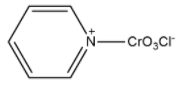
(B)
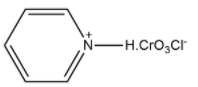
(C)

(D)
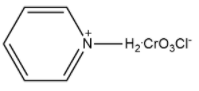




Answer
582.6k+ views
Hint: Try to recollect the structure of Pyridinium chlorochromate (PCC). It is used as a catalyst for selective oxidation of alcohols to aldehydes. Start by writing the structure of pyridine and then write the molecular formula of chlorochromate ion. Then try drawing the structure of PCC to get the answer.
Complete step by step solution:
- Pyridinium chlorochromate is yellow-orange salt and is abbreviated as PCC.
- PCC is used as a catalyst in selective oxidation of primary alcohols to form aldehydes and secondary alcohols to form ketones.
- PCC is a mild oxidizing agent and doesn’t oxidize aldehydes and ketones to carboxylic acids.
- Pyridine is a six-membered heterocyclic compound having one nitrogen atom and five carbon atoms. Pyridinium is a cation of pyridine formed when the nitrogen atom donates its electron to hydrogen thereby developing a positive charge.
- Chlorochromate is an anion in which one of the oxygen atoms in chromate group, $CrO_{4}^{2-}$ is replaced by one chlorine atom to form, $Cr{{O}_{3}}C{{l}^{-}}$.
- PCC is a salt containing pyridinium cation and chlorochromate ion and therefore has the structure as given below.
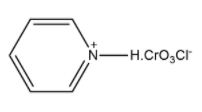
Therefore, the correct structure of Pyridinium chlorochromate is option (B).
Note: Remember Pyridinium chlorochromate (PCC) and Pyridinium dichromate (PDC) are mild oxidizing agents which oxidize alcohols to aldehydes or ketones. They do not oxidize aldehydes or ketones to carboxylic acids and therefore, they are used in the synthesis of carbonyl compounds from alcohols.
Complete step by step solution:
- Pyridinium chlorochromate is yellow-orange salt and is abbreviated as PCC.
- PCC is used as a catalyst in selective oxidation of primary alcohols to form aldehydes and secondary alcohols to form ketones.
- PCC is a mild oxidizing agent and doesn’t oxidize aldehydes and ketones to carboxylic acids.
- Pyridine is a six-membered heterocyclic compound having one nitrogen atom and five carbon atoms. Pyridinium is a cation of pyridine formed when the nitrogen atom donates its electron to hydrogen thereby developing a positive charge.
- Chlorochromate is an anion in which one of the oxygen atoms in chromate group, $CrO_{4}^{2-}$ is replaced by one chlorine atom to form, $Cr{{O}_{3}}C{{l}^{-}}$.
- PCC is a salt containing pyridinium cation and chlorochromate ion and therefore has the structure as given below.

Therefore, the correct structure of Pyridinium chlorochromate is option (B).
Note: Remember Pyridinium chlorochromate (PCC) and Pyridinium dichromate (PDC) are mild oxidizing agents which oxidize alcohols to aldehydes or ketones. They do not oxidize aldehydes or ketones to carboxylic acids and therefore, they are used in the synthesis of carbonyl compounds from alcohols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




