
Why is ice a crystalline compound?
Answer
507k+ views
Hint: Solids are of two types: 1) Crystalline and 2) amorphous. In crystalline solids, atoms are present in long range orderly arrangement while amorphous solids contain short range orderly arrangement of atoms. The smallest cell which is repeated in three dimensions to form crystalline solid is called unit cell.
Complete answer:
When water solidifies, ice is formed. This process is called solidification. The formed ice is a crystalline compound because water molecules are arranged in long range orderly arrangement which ultimately lead to cage-like structure. This is illustrate in the following diagram-
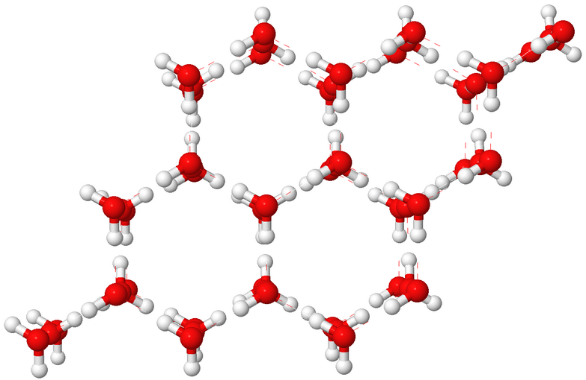
In the above diagram, the red circle represents the oxygen atom and white circle represents the hydrogen atom. In cage-like structure, each water molecule is tetrahedrally bonded to four other water molecules via hydrogen bonding.
Additional Information:
There are several points of difference between crystalline and amorphous solids. Crystalline solids have sharp melting points and are anisotropic in nature while amorphous melts over a range of temperature and is isotropic in nature. Anisotropy means different values of several properties occur when measurement is done in different directions. Moreover, the Crystallization process is used in labs in order to form pure crystals from the mother liquor. X-ray crystallography is a technique which is used to determine the structure of a crystal.
Note:
It is important to note that ice is a crystalline compound because of the long range orderly arrangement of water molecules. This long range order arrangement leads to the cage-like structure. Each water molecule is tetrahedrally bonded to four other water molecules via hydrogen bonding.
Complete answer:
When water solidifies, ice is formed. This process is called solidification. The formed ice is a crystalline compound because water molecules are arranged in long range orderly arrangement which ultimately lead to cage-like structure. This is illustrate in the following diagram-

In the above diagram, the red circle represents the oxygen atom and white circle represents the hydrogen atom. In cage-like structure, each water molecule is tetrahedrally bonded to four other water molecules via hydrogen bonding.
Additional Information:
There are several points of difference between crystalline and amorphous solids. Crystalline solids have sharp melting points and are anisotropic in nature while amorphous melts over a range of temperature and is isotropic in nature. Anisotropy means different values of several properties occur when measurement is done in different directions. Moreover, the Crystallization process is used in labs in order to form pure crystals from the mother liquor. X-ray crystallography is a technique which is used to determine the structure of a crystal.
Note:
It is important to note that ice is a crystalline compound because of the long range orderly arrangement of water molecules. This long range order arrangement leads to the cage-like structure. Each water molecule is tetrahedrally bonded to four other water molecules via hydrogen bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




