
Ibuprofen is a
E. propanoic acid derivative
F. benzoic acid derivative
G. naphthoic acid derivative
H. sulphanilic acid derivative
Answer
573.3k+ views
Hint: Ibuprofen is a nonsteroidal anti-inflammatory (NSAID) drug which is used in treating pain and fever as it is a non-narcotic analgesic. It is a monocarboxylic acid in which one of the hydrogen is at a second position and is substituted by a 4-phenyl group.
Complete step by step solution:
As the structure of ibuprofen has three carbon chains which has different groups attached to it. It is an acid derivative of propanoic acid as it is derived from propanoic acid. The three carbons present in the same chain are called propane for single bond and propene for double bond.
The Formula for ibuprofen is \[{C_{13}}{H_{18}}{O_2}\] and is a chiral compound or we can say that is a racemic mixture. It is very less or not at all soluble in water as the Solubility in water is \[0.021{\text{ }}mg/mL\] at $20^\circ C$ temperature.
Ibuprofen was derived from propionic acid through the efforts of scientists of the research arm of the Boots Group. The name of ibuprofen is derived by the presence of 3 functional groups that are isobutyl (ibu), propionic acid (pro) and phenyl (fen).
Hence, Ibuprofen is a propanoic acid derivative.
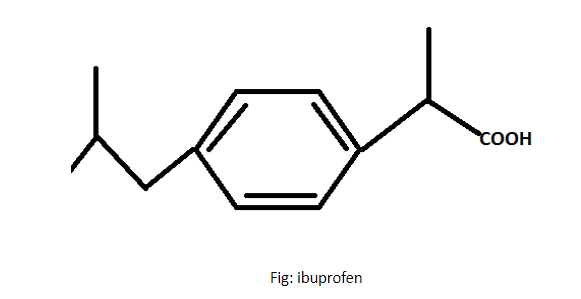
Therefore the correct option is (A).
Note: There are chemical and physical properties of Ibuprofen, it is nearly insoluble in water, but it is easily soluble in most of the organic solvents such as ethanol methanol, acetone and others. The Density of it is \[1.03{\text{ }}g/ml{\text{ }}g/cm3\]. Melting point is \[75{\text{ }}to{\text{ }}78{\text{ }}^\circ C\] and the Boiling point is \[157{\text{ }}^\circ C\]. Ibuprofen is also called isobutylphenyl propionic acid.
Complete step by step solution:
As the structure of ibuprofen has three carbon chains which has different groups attached to it. It is an acid derivative of propanoic acid as it is derived from propanoic acid. The three carbons present in the same chain are called propane for single bond and propene for double bond.
The Formula for ibuprofen is \[{C_{13}}{H_{18}}{O_2}\] and is a chiral compound or we can say that is a racemic mixture. It is very less or not at all soluble in water as the Solubility in water is \[0.021{\text{ }}mg/mL\] at $20^\circ C$ temperature.
Ibuprofen was derived from propionic acid through the efforts of scientists of the research arm of the Boots Group. The name of ibuprofen is derived by the presence of 3 functional groups that are isobutyl (ibu), propionic acid (pro) and phenyl (fen).
Hence, Ibuprofen is a propanoic acid derivative.

Therefore the correct option is (A).
Note: There are chemical and physical properties of Ibuprofen, it is nearly insoluble in water, but it is easily soluble in most of the organic solvents such as ethanol methanol, acetone and others. The Density of it is \[1.03{\text{ }}g/ml{\text{ }}g/cm3\]. Melting point is \[75{\text{ }}to{\text{ }}78{\text{ }}^\circ C\] and the Boiling point is \[157{\text{ }}^\circ C\]. Ibuprofen is also called isobutylphenyl propionic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




