
Hydrogen peroxide molecules are:
A.Monatomic and form $X_2^{2 - }$ ions
B.Diatomic and form $X_{}^ - $ ions
C.Diatomic and form $HO_2^ - $ ions
D.Monoatomic and form $X_{}^ - $ ions
Answer
573k+ views
Hint:To answer this question, you should recall the properties of hydrogen peroxide. The chemical formula for hydrogen peroxide is ${H_2}{O_2}$. It is symmetrical and each OH ion has a negative charge.
Complete step by step solution:
We know that peroxide is a compound which has two oxygen atoms bonded together. And Hydrogen Peroxide is the simplest peroxide. The structure of the molecule can be drawn as:
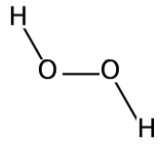
Hydrogen peroxide is a colorless liquid and is used as a bleaching agent and disinfectant due to its property to produce free radicals on decomposition. Hydrogen peroxide decomposes when exposed to sunlight, hence it is stored in wax-lined glass or plastic containers and kept in dark. This process is catalyzed and accelerated by traces of alkali metals. It should also be kept away from dust particles because dust can induce explosive decomposition of this compound. Hydrogen peroxide is both acidic and the basic medium acts as an oxidizing as well as the reducing agent\[{H_2}{O_2}\] is a monatomic molecule as it contains only one atom. It can be simply represented as \[HO - OH.\] It forms \[{\left( {OH} \right)_2}^{2 - }\] ions on breakage.
That is, it forms \[{X_2}^{2 - }\] ions. \[HO - OH \rightleftharpoons {\left( {OH} \right)_2}^{ - 2}\].
Hence, the correct option is option A.
Note:
You should know about the preparation of Hydrogen Peroxide:
\[N{a_2}{O_2}\left( s \right) + {H_2}S{O_4} \to N{a_2}S{O_4}\left( s \right) + {H_2}{O_2}\left( l \right)\].
We can see that here \[{H_2}{O_2}\]is being produced. This is indeed a preparation method of laboratory preparation of hydrogen peroxide by the action of ice-cold, dilute sulphuric acid on sodium peroxide or hydrated barium peroxide. A calculated quantity of sodium peroxide is added in small quantities to $20\% $an ice-cold solution of sulphuric acid. Most of the sodium sulphate separates on cooling as crystals of \[N{a_2}S{O_4}.10{H_2}O\]. From this reaction, we obtain a \[30\% \] hydrogen peroxide yield containing a small amount of sodium sulphate.
Complete step by step solution:
We know that peroxide is a compound which has two oxygen atoms bonded together. And Hydrogen Peroxide is the simplest peroxide. The structure of the molecule can be drawn as:

Hydrogen peroxide is a colorless liquid and is used as a bleaching agent and disinfectant due to its property to produce free radicals on decomposition. Hydrogen peroxide decomposes when exposed to sunlight, hence it is stored in wax-lined glass or plastic containers and kept in dark. This process is catalyzed and accelerated by traces of alkali metals. It should also be kept away from dust particles because dust can induce explosive decomposition of this compound. Hydrogen peroxide is both acidic and the basic medium acts as an oxidizing as well as the reducing agent\[{H_2}{O_2}\] is a monatomic molecule as it contains only one atom. It can be simply represented as \[HO - OH.\] It forms \[{\left( {OH} \right)_2}^{2 - }\] ions on breakage.
That is, it forms \[{X_2}^{2 - }\] ions. \[HO - OH \rightleftharpoons {\left( {OH} \right)_2}^{ - 2}\].
Hence, the correct option is option A.
Note:
You should know about the preparation of Hydrogen Peroxide:
\[N{a_2}{O_2}\left( s \right) + {H_2}S{O_4} \to N{a_2}S{O_4}\left( s \right) + {H_2}{O_2}\left( l \right)\].
We can see that here \[{H_2}{O_2}\]is being produced. This is indeed a preparation method of laboratory preparation of hydrogen peroxide by the action of ice-cold, dilute sulphuric acid on sodium peroxide or hydrated barium peroxide. A calculated quantity of sodium peroxide is added in small quantities to $20\% $an ice-cold solution of sulphuric acid. Most of the sodium sulphate separates on cooling as crystals of \[N{a_2}S{O_4}.10{H_2}O\]. From this reaction, we obtain a \[30\% \] hydrogen peroxide yield containing a small amount of sodium sulphate.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




