
How many hydrogen bonded water molecules are associated with $CuS{{O}_{4}}.5{{H}_{2}}O$?
A. five
B. one
C. four
D. three
Answer
564.9k+ views
Hint: In order to find hydrogen bonded water molecules are associated with $CuS{{O}_{4}}.5{{H}_{2}}O$, we will first draw a structure of copper sulphate $CuS{{O}_{4}}.5{{H}_{2}}O$ and then will check out hydrogen bonds present in copper sulphate.
Complete Solution :
- We can draw the structure of $CuS{{O}_{4}}.5{{H}_{2}}O$ as:
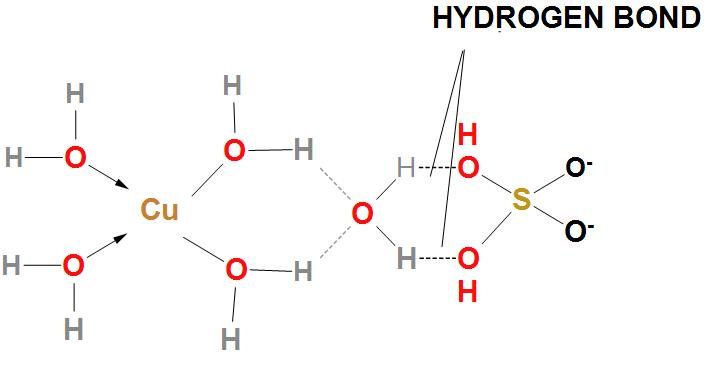
- Here, in structure we can find out that there are five water molecules , out of these five molecules of water, four water molecules are coordinated to $C{{u}^{+2}}$, whereas one is hydrogen bonded with $S{{O}_{4}}^{-}$.
- Here, we can see that copper is bonded to two oxygen atoms from two sulphate and four water molecules in a square planar geometry. Four water molecules are coordinated with $C{{u}^{+2}}$ ion.
- Whereas, the fifth water molecule is hydrogen bonded, and is not coordinated. We can say that four water molecules are coordinated and one water molecule is hydrogen bonded.
- Hence, we can conclude that the correct option is (b), that is one hydrogen bonded water molecules are associated with $CuS{{O}_{4}}.5{{H}_{2}}O$
So, the correct answer is “Option B”.
Note: - We must know that covalent chemical bond is also called a coordinated bond, which is formed when one atom shares a pair of electrons with another atom, lacking such a pair then the formation of bond that occurs is covalent chemical bond.
- After drawing the structure, we can check whether the shared pair of electrons are coming from the same molecule or not. And if we found that it comes from the same molecule then the compound contains a coordinated bond.
Complete Solution :
- We can draw the structure of $CuS{{O}_{4}}.5{{H}_{2}}O$ as:

- Here, in structure we can find out that there are five water molecules , out of these five molecules of water, four water molecules are coordinated to $C{{u}^{+2}}$, whereas one is hydrogen bonded with $S{{O}_{4}}^{-}$.
- Here, we can see that copper is bonded to two oxygen atoms from two sulphate and four water molecules in a square planar geometry. Four water molecules are coordinated with $C{{u}^{+2}}$ ion.
- Whereas, the fifth water molecule is hydrogen bonded, and is not coordinated. We can say that four water molecules are coordinated and one water molecule is hydrogen bonded.
- Hence, we can conclude that the correct option is (b), that is one hydrogen bonded water molecules are associated with $CuS{{O}_{4}}.5{{H}_{2}}O$
So, the correct answer is “Option B”.
Note: - We must know that covalent chemical bond is also called a coordinated bond, which is formed when one atom shares a pair of electrons with another atom, lacking such a pair then the formation of bond that occurs is covalent chemical bond.
- After drawing the structure, we can check whether the shared pair of electrons are coming from the same molecule or not. And if we found that it comes from the same molecule then the compound contains a coordinated bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




