
What is the hybridization of boron in ${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ ?
A.${\text{s}}{{\text{p}}^{\text{2}}}$
B.${\text{s}}{{\text{p}}^3}$
C.${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$
D.None of these
Answer
577.8k+ views
Hint: Hybridization refers to the intermixing of two or more atomic orbitals which are of nearly the same energies to give rise to the formation of new orbitals called hybrid orbitals.
Hybridization takes place in the following ways: ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization, ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridization, sp hybridization, ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$ hybridization and ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ hybridization.
Boron is a second period or first row element and so it does not have vacant d orbitals to undergo ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ hybridization.
Complete step by step answer:
We need to find out the hybridization of boron atom in the ion${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ .
When the 2s and the three 2p orbitals of boron, viz. the 2px, 2py and 2pz orbitals get intermixed, four new orbitals called the${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid orbitals will be formed. An ${\text{s}}{{\text{p}}^{\text{3}}}$hybridIzed orbital has s-character 25% and p-character 75%.
In ${\text{s}}{{\text{p}}^{\text{2}}}$hybridization, the 2s orbital and two of the three 2p orbitals of boron get intermixed, while the third 2p orbital remains unchanged. A ${\text{s}}{{\text{p}}^{\text{2}}}$hybrid orbital has 33% s-character and 66% p-character. In sp hybridization, the 2s orbital and one of the three 2p orbitals of boron get intermixed, while the remaining two 2p orbitals are left unchanged.
Now, let us study the structure of the ${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ ion.
Boron atom has the atomic number 5 and so in the ground state has the electronic configuration \[{\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{1}}}\] .
Boron atom in the first excited state has the electronic configuration has has the electronic configuration \[{\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^1}{\text{2}}{{\text{p}}_{\text{x}}}^{\text{1}}{\text{2}}{{\text{p}}_{\text{y}}}^{\text{1}}\] . Thus, boron forms 4 ${\text{s}}{{\text{p}}^{\text{3}}}$hybridized orbitals.
In the ${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ ion, the unpaired orbitals will form 3 bonds with 3 hydroxyl groups. The vacant orbital forms a coordinate bond with the ${\text{O}}{{\text{H}}^ - }$ group.
Thus, boron will have ${\text{s}}{{\text{p}}^{\text{3}}}$hybridization in ${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ and the molecule will have tetrahedral structure.
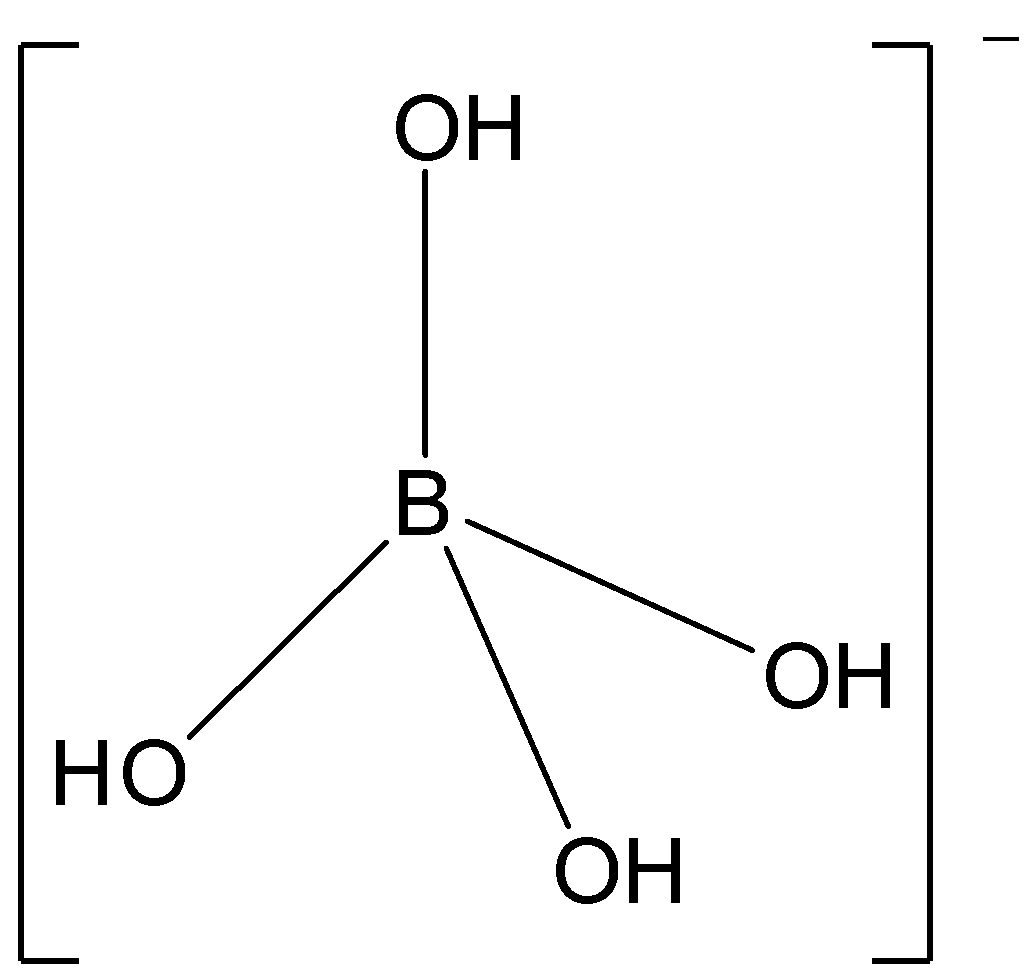
So, option B is correct.
Note:
Tetrahydroxyborate is produced when boric acid and hydroxide ions react with each other.
${\text{B}}{\left( {{\text{OH}}} \right)_{\text{3}}}{\text{ + H}}{{\text{O}}^{\text{ - }}} \to {\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^ - }$
It can again capture a proton and assimilate it into the anion to form boric acid because of which it has a weak basic character.
${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^ - } + {{\text{H}}^ + } \to {\text{B}}{\left( {{\text{OH}}} \right)_{\text{3}}}{\text{ + }}{{\text{H}}_2}{\text{O}}$
Hybridization takes place in the following ways: ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization, ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridization, sp hybridization, ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$ hybridization and ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ hybridization.
Boron is a second period or first row element and so it does not have vacant d orbitals to undergo ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ hybridization.
Complete step by step answer:
We need to find out the hybridization of boron atom in the ion${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ .
When the 2s and the three 2p orbitals of boron, viz. the 2px, 2py and 2pz orbitals get intermixed, four new orbitals called the${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid orbitals will be formed. An ${\text{s}}{{\text{p}}^{\text{3}}}$hybridIzed orbital has s-character 25% and p-character 75%.
In ${\text{s}}{{\text{p}}^{\text{2}}}$hybridization, the 2s orbital and two of the three 2p orbitals of boron get intermixed, while the third 2p orbital remains unchanged. A ${\text{s}}{{\text{p}}^{\text{2}}}$hybrid orbital has 33% s-character and 66% p-character. In sp hybridization, the 2s orbital and one of the three 2p orbitals of boron get intermixed, while the remaining two 2p orbitals are left unchanged.
Now, let us study the structure of the ${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ ion.
Boron atom has the atomic number 5 and so in the ground state has the electronic configuration \[{\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{1}}}\] .
Boron atom in the first excited state has the electronic configuration has has the electronic configuration \[{\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^1}{\text{2}}{{\text{p}}_{\text{x}}}^{\text{1}}{\text{2}}{{\text{p}}_{\text{y}}}^{\text{1}}\] . Thus, boron forms 4 ${\text{s}}{{\text{p}}^{\text{3}}}$hybridized orbitals.
In the ${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ ion, the unpaired orbitals will form 3 bonds with 3 hydroxyl groups. The vacant orbital forms a coordinate bond with the ${\text{O}}{{\text{H}}^ - }$ group.
Thus, boron will have ${\text{s}}{{\text{p}}^{\text{3}}}$hybridization in ${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^{\text{ - }}}$ and the molecule will have tetrahedral structure.

So, option B is correct.
Note:
Tetrahydroxyborate is produced when boric acid and hydroxide ions react with each other.
${\text{B}}{\left( {{\text{OH}}} \right)_{\text{3}}}{\text{ + H}}{{\text{O}}^{\text{ - }}} \to {\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^ - }$
It can again capture a proton and assimilate it into the anion to form boric acid because of which it has a weak basic character.
${\left[ {{\text{B}}{{\left( {{\text{OH}}} \right)}_{\text{4}}}} \right]^ - } + {{\text{H}}^ + } \to {\text{B}}{\left( {{\text{OH}}} \right)_{\text{3}}}{\text{ + }}{{\text{H}}_2}{\text{O}}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




