
What is the hybridization in the central atom of ${{H}_{3}}O+$?
Answer
507.3k+ views
Hint: The central atom in ${{H}_{3}}O+$is Oxygen. It loses one electron to gain a positive charge. ${{H}_{3}}O+$is the cation as it has one positive charge on it. The hybridization of a molecule depends on the number of lone pairs and bond pairs present on the atom.
Complete answer:
To understand it easily, first, let us see the structure of ${{H}_{3}}O+$ ion.
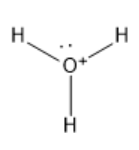
We know that Oxygen is the central atom in the hydronium ion. It has three hydrogen atoms attached to it via a single bond.
As per the electronic configuration of Oxygen, it has 6 electrons in the last shell, but it loses one electron and gains a positive charge. Thus, there are 5 electrons left in the last shell which forms a lone pair of electrons.
To find the hybridization of ${{H}_{3}}O+$ we need to calculate the number of electrons present in the ion.
The formula is: $\text{Hybridization (No}\text{. of electrons) = }\dfrac{1}{2}[V+M-C+A]$
We can directly identify the hybridization in a molecule by just knowing the number of electrons.
Now let’s calculate the number of electrons:
$\begin{align}
& \text{No}\text{. of electrons = }\dfrac{1}{2}[V+M-C+A] \\
& \\
\end{align}$
Here,
V= No. of valence electrons in central atom = 6
M = No. of monovalent atoms attached to central atom = 3
C = Charge on cation = 1
A = Charge on Anion = 0
$\begin{align}
& \text{No}\text{. of electrons = }\dfrac{1}{2}[6+3-1+0] \\
& =\text{ }\dfrac{1}{2}[9-1] \\
& =\text{ }\dfrac{1}{2}[8] \\
& =4 \\
\end{align}$
The number of electrons is 4, so the hybridization will be $s{{p}^{3}}$hybridization. With an ion having 4 electrons the electronic geometry comes to be tetrahedral.
Note:
To understand it in an easier way, draw the Lewis dot structure of hydronium ions. The structure shows four pairs of electrons that are present in the atom. Out of these three pairs are shared with hydrogen atoms to form bonds and the fourth electron pair is the lone pair.
Complete answer:
To understand it easily, first, let us see the structure of ${{H}_{3}}O+$ ion.

We know that Oxygen is the central atom in the hydronium ion. It has three hydrogen atoms attached to it via a single bond.
As per the electronic configuration of Oxygen, it has 6 electrons in the last shell, but it loses one electron and gains a positive charge. Thus, there are 5 electrons left in the last shell which forms a lone pair of electrons.
To find the hybridization of ${{H}_{3}}O+$ we need to calculate the number of electrons present in the ion.
The formula is: $\text{Hybridization (No}\text{. of electrons) = }\dfrac{1}{2}[V+M-C+A]$
We can directly identify the hybridization in a molecule by just knowing the number of electrons.
| No of electrons | Hybridization |
| 2 | $sp$ |
| 3 | $s{{p}^{2}}$ |
| 4 | $s{{p}^{3}}$ |
| 5 | $s{{p}^{3}}d$ |
| 6 | $s{{p}^{3}}{{d}^{2}}$ |
Now let’s calculate the number of electrons:
$\begin{align}
& \text{No}\text{. of electrons = }\dfrac{1}{2}[V+M-C+A] \\
& \\
\end{align}$
Here,
V= No. of valence electrons in central atom = 6
M = No. of monovalent atoms attached to central atom = 3
C = Charge on cation = 1
A = Charge on Anion = 0
$\begin{align}
& \text{No}\text{. of electrons = }\dfrac{1}{2}[6+3-1+0] \\
& =\text{ }\dfrac{1}{2}[9-1] \\
& =\text{ }\dfrac{1}{2}[8] \\
& =4 \\
\end{align}$
The number of electrons is 4, so the hybridization will be $s{{p}^{3}}$hybridization. With an ion having 4 electrons the electronic geometry comes to be tetrahedral.
Note:
To understand it in an easier way, draw the Lewis dot structure of hydronium ions. The structure shows four pairs of electrons that are present in the atom. Out of these three pairs are shared with hydrogen atoms to form bonds and the fourth electron pair is the lone pair.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




