
Hybridization and geometry of ${{\text{[Ni(CN}}{{\text{)}}_{\text{4}}}{\text{]}}^{{\text{2 - }}}}$ are:
a.) ${\text{s}}{{\text{p}}^{\text{2}}}{\text{d}}$ and tetrahedral
b.) ${\text{s}}{{\text{d}}^{\text{3}}}$and square planar
c.) ${\text{s}}{{\text{p}}^{\text{3}}}$ and tetrahedral
d.) ${\text{ds}}{{\text{p}}^{\text{2}}}$ and square planar
Answer
599.4k+ views
Hint: If we know the concept of hybridization, then we can easily find out the geometry of the compound. After drawing the excited electronic configuration, we can find out the hybridization easily. Also, there is a particular geometry associated with a type of hybridization.
Complete step by step answer:
First of all we have to find out the oxidation state of central metal atom Ni. That is, $x + (4 \times (- 1)) = - 2 \Rightarrow x = +2$. Therefore the oxidation state of Nickel is +2. So, let us draw subshell electronic configuration of ${\text{N}}{{\text{i}}^{{\text{ + 2}}}}$:
${\text{N}}{{\text{i}}^{{\text{ + 2}}}}$: ${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{8}}}$
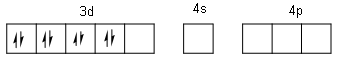
Here, one 3d orbital, one 4s orbital and three 4p orbitals are free. Out of which we need four orbitals because we have four ${\text{C}}{{\text{N}}^{\text{ - }}}$ligands. So, the four orbitals are one 3d, one 4s and two 4p. Therefore, the hybridization is ${\text{ds}}{{\text{p}}^{\text{2}}}$. For the formation of square planar geometry, two unpaired d-electrons are paired up due to energy made by the approach of ligands, making one of the 3d orbitals empty.
So, the hybridization is ${\text{ds}}{{\text{p}}^{\text{2}}}$ and the geometry is square planar. Therefore, the correct option is (d).
Additional Information:
We know that hybridization is the concept of mixing two atomic orbitals with the same energy levels to give a degenerated new type of orbitals. This intermixing is based on quantum mechanics.
The atomic orbitals having the same energy level can only take part in hybridization and both full filled and half filled orbitals can also take part in this process.
Note: Whenever you are drawing the electronic configuration of the central metal atom, you need to pair up the unpaired electrons in the presence of strong ligands, otherwise you will not get the right hybridization.
Complete step by step answer:
First of all we have to find out the oxidation state of central metal atom Ni. That is, $x + (4 \times (- 1)) = - 2 \Rightarrow x = +2$. Therefore the oxidation state of Nickel is +2. So, let us draw subshell electronic configuration of ${\text{N}}{{\text{i}}^{{\text{ + 2}}}}$:
${\text{N}}{{\text{i}}^{{\text{ + 2}}}}$: ${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{8}}}$

Here, one 3d orbital, one 4s orbital and three 4p orbitals are free. Out of which we need four orbitals because we have four ${\text{C}}{{\text{N}}^{\text{ - }}}$ligands. So, the four orbitals are one 3d, one 4s and two 4p. Therefore, the hybridization is ${\text{ds}}{{\text{p}}^{\text{2}}}$. For the formation of square planar geometry, two unpaired d-electrons are paired up due to energy made by the approach of ligands, making one of the 3d orbitals empty.
So, the hybridization is ${\text{ds}}{{\text{p}}^{\text{2}}}$ and the geometry is square planar. Therefore, the correct option is (d).
Additional Information:
We know that hybridization is the concept of mixing two atomic orbitals with the same energy levels to give a degenerated new type of orbitals. This intermixing is based on quantum mechanics.
The atomic orbitals having the same energy level can only take part in hybridization and both full filled and half filled orbitals can also take part in this process.
Note: Whenever you are drawing the electronic configuration of the central metal atom, you need to pair up the unpaired electrons in the presence of strong ligands, otherwise you will not get the right hybridization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




