
Hybridised states of Carbon in Graphite and Diamond respectively are….
(A) \[s{p^3}\] , \[s{p^3}\]
(B) \[s{p^3}\] , \[s{p^2}\]
(C) \[s{p^2}\] , \[s{p^2}\]
(D) \[s{p^2}\] , \[s{p^3}\]
Answer
598.2k+ views
Hint: Graphite has a Trigonal Planar geometry while Diamond has a Tetragonal geometry. In Graphite, hybrid orbitals have 33% s-character and 66% p-character. Hybrid orbitals in Diamond have 25% s-character and 75% p-character.
Complete Step by Step solution:
Now, let's see the structure of both Graphite and Diamond.
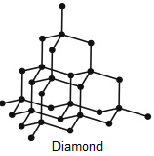
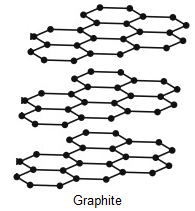
Here, We can see in Diamond that each carbon atom is bonded with 4 carbon atoms and in Graphite, each carbon atom is bonded with other 3 carbon atoms. (That is a fact and needs to be remembered !) Also the structure does not involve any other atoms than Carbon.
Now Firstly, let's see Graphite. In Graphite, the Carbon-Carbon bond angle is 120\[^\circ \]also there is Trigonal Planar geometry in the molecule. So, it matches the characteristics of \[s{p^2}\] hybridisation. Here, one carbon is having 2 single bonds and a double bond and that completes the valency of Carbon which is 4. This proves that it has \[s{p^2}\] hybridization.
In Diamond, Each Carbon atom is bonded with other 4 carbon atoms. All the Carbon-Carbon bonds are single bonds here. Also, Geometry is Tetragonal having bond angles 109\[^\circ \]28’. So, this matches with the characteristics of \[s{p^3}\] hybridisation.
So, Correct answer is (D) \[s{p^2}\] , \[s{p^3}\]
Additional Information:
Both Diamond and Graphite are allotropes of Carbon. They have only Carbon atoms in their structure. Both have different structures and different characteristics. Lets know the basics about \[s{p^2}\] and \[s{p^3}\] hybridisation.
In \[s{p^2}\] hybridisation, one s-orbital and two p-orbital form 3 \[\sigma \] bonds while the other p-orbital overlaps and forms a \[\pi \] bond.
In \[s{p^3}\] hybridisation, one s-orbital and three p-orbitals bind to form 4 \[\sigma \] bonds.
Note: One should know the structure and geometry of Diamond and Graphite to proceed further to find out the hybridization. Also these structures contain only carbon atoms hence it makes it easy to judge the hybridization by bond angles, shape and structure. If different atoms were present in the compound and it has complex structure then only we go and try to find hybridization by putting electrons in respective orbitals to make things easier.
Complete Step by Step solution:
Now, let's see the structure of both Graphite and Diamond.


Here, We can see in Diamond that each carbon atom is bonded with 4 carbon atoms and in Graphite, each carbon atom is bonded with other 3 carbon atoms. (That is a fact and needs to be remembered !) Also the structure does not involve any other atoms than Carbon.
Now Firstly, let's see Graphite. In Graphite, the Carbon-Carbon bond angle is 120\[^\circ \]also there is Trigonal Planar geometry in the molecule. So, it matches the characteristics of \[s{p^2}\] hybridisation. Here, one carbon is having 2 single bonds and a double bond and that completes the valency of Carbon which is 4. This proves that it has \[s{p^2}\] hybridization.
In Diamond, Each Carbon atom is bonded with other 4 carbon atoms. All the Carbon-Carbon bonds are single bonds here. Also, Geometry is Tetragonal having bond angles 109\[^\circ \]28’. So, this matches with the characteristics of \[s{p^3}\] hybridisation.
So, Correct answer is (D) \[s{p^2}\] , \[s{p^3}\]
Additional Information:
Both Diamond and Graphite are allotropes of Carbon. They have only Carbon atoms in their structure. Both have different structures and different characteristics. Lets know the basics about \[s{p^2}\] and \[s{p^3}\] hybridisation.
In \[s{p^2}\] hybridisation, one s-orbital and two p-orbital form 3 \[\sigma \] bonds while the other p-orbital overlaps and forms a \[\pi \] bond.
In \[s{p^3}\] hybridisation, one s-orbital and three p-orbitals bind to form 4 \[\sigma \] bonds.
Note: One should know the structure and geometry of Diamond and Graphite to proceed further to find out the hybridization. Also these structures contain only carbon atoms hence it makes it easy to judge the hybridization by bond angles, shape and structure. If different atoms were present in the compound and it has complex structure then only we go and try to find hybridization by putting electrons in respective orbitals to make things easier.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




