
What hybrid orbitals are employed by carbon atom ${{1, 2 and 3}}$respectively as labelled in the compound shown?
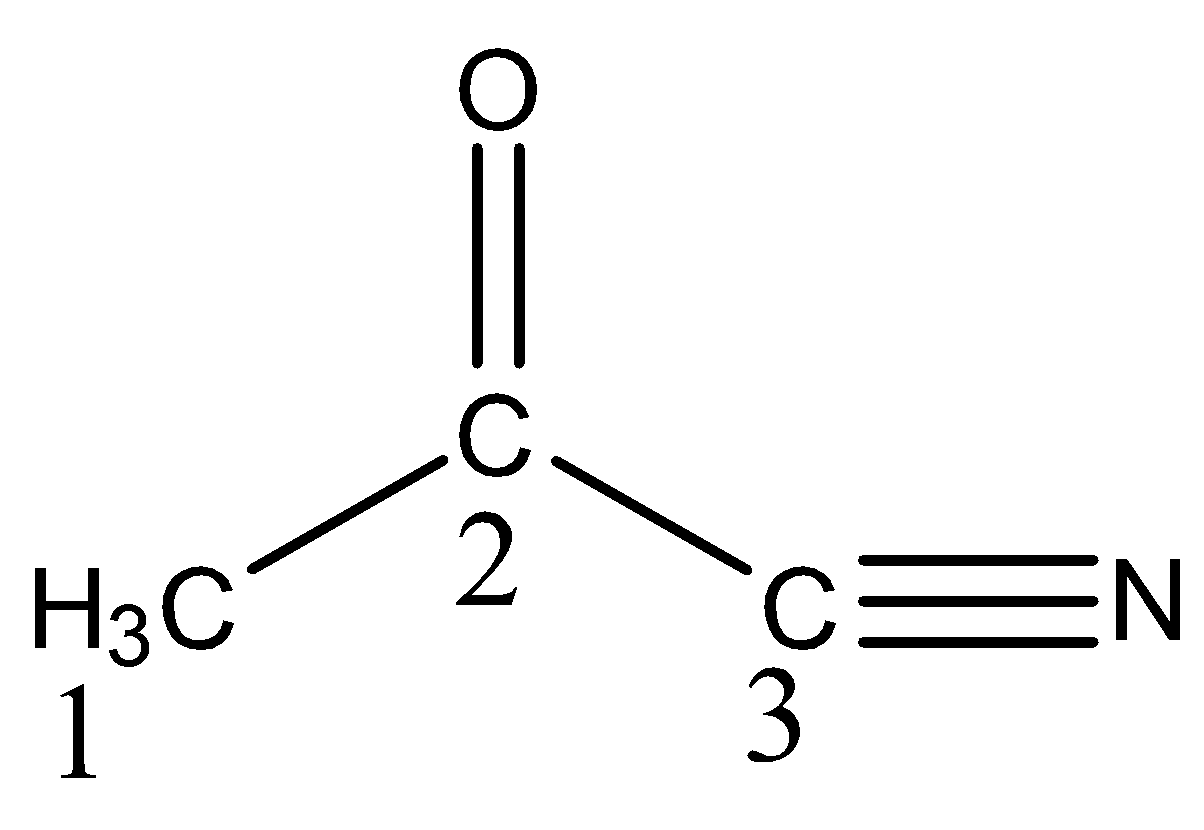
(A) ${{s}}{{{p}}^{{3}}}{{, sp , sp}}$
(B) ${{s}}{{{p}}^{{2}}}{{ , s}}{{{p}}^{{3}}}{{ , sp}}$
(C) ${{s}}{{{p}}^{{3}}}{{ , s}}{{{p}}^{{2}}}{{ , sp}}$
(D) ${{s}}{{{p}}^{{3}}}{{ , s}}{{{p}}^{{2}}}{{ , s}}{{{p}}^{{2}}}$

Answer
570k+ views
Hint: First we will see the number of bonds between the carbon and its substituents and see the type of overlapping of the orbitals involved in the formation of the compound. Every hybridized orbital has a different shape than the pure orbital.
Complete step by step answer:
First, we will see the type of overlapping and shape of the hybridized orbitals.
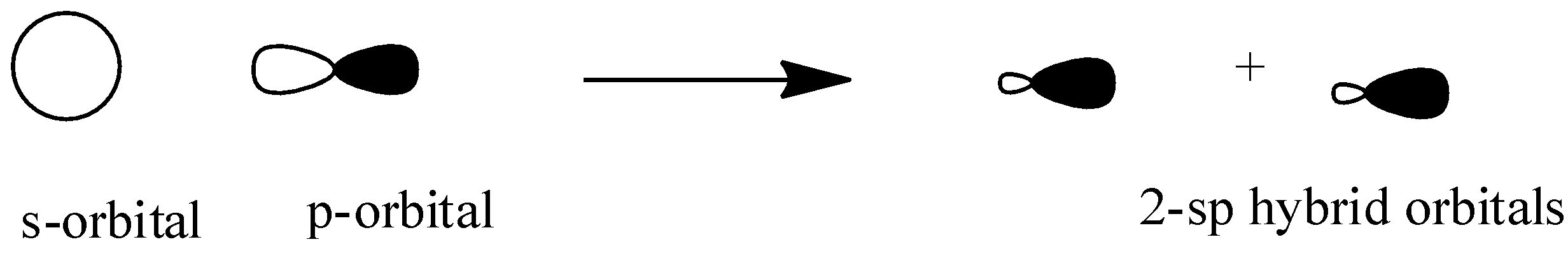
\[{{sp}}\]: The \[{{sp}}\] hybridization occurs when one s-orbital and one p-orbital overlap each other head on to form two \[{{sp}}\] hybridized orbitals having linear shape. Here two p-orbitals are unhybridized so they form ${{2\pi }}$ bonds.
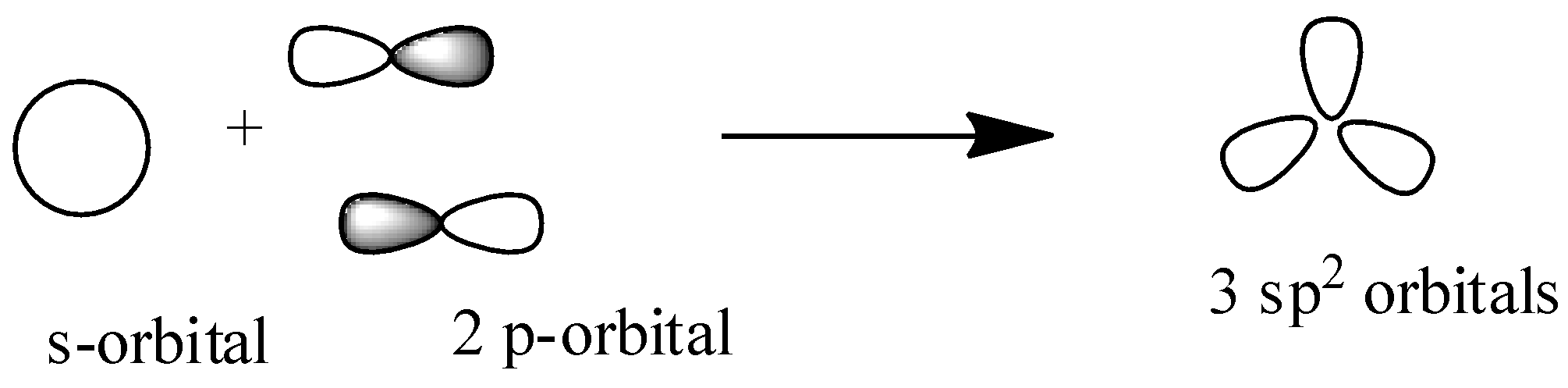
${{s}}{{{p}}^{{2}}}$: Here in this hybridization one s-orbital and two p-orbitals overlap to form ${{3 s}}{{{p}}^{{2}}}$ hybridized orbitals and here one p-orbital is unhybridized so that it can form ${{\pi }}$-bond between two carbon or between carbon and a heteroatom. So ${{s}}{{{p}}^{{2}}}$ hybridized orbital has ${{3\sigma orbitals and 1\pi orbital}}$ to make single and double bond. It has trigonal shape
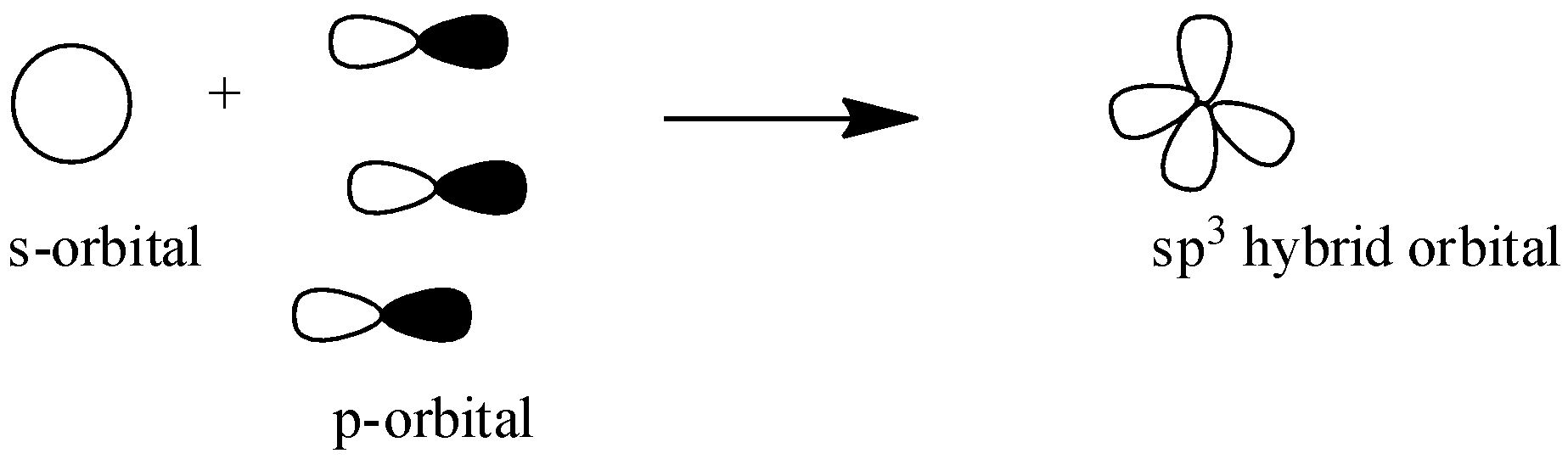
${{s}}{{{p}}^{{3}}}$ : In this hybridization one s-orbital overlaps with three p-orbital to form three ${{s}}{{{p}}^{{3}}}$ orbitals. Here we don’t have any unhybridized p-orbital to form ${{ \pi }}$ bond only ${{4\sigma }}$ bonds are formed and structure of these hybridized orbitals are tetrahedron.
So now in structure carbon ${{1}}$ has ${{4\sigma\; bonds\; no \;\pi \; bond}}$ so the hybridization of carbon 1 is $sp^3$
When we see carbon ${{2}}$ we observe it has ${{3\sigma \; and \; 1\pi \;bond}}$ and has trigonal planar structure so this fits the condition for ${{s}}{{{p}}^{{2}}}$ hybridization.
Now last carbon ${{3}}$ we see it has ${{2\sigma \; and \;2\pi \; bonds}}$ in it and structure is also linear then the hybridization is sp
So, the correct answer is Option C .
Note: By knowing the hybridization of the carbon or any element in a molecule we can know the nature of bond and characteristic of it whether the bond is strong or weak or it makes the molecule electron donating or withdrawing group.
The triple bond is the strongest among the single , double and triple bond because single bond only have ${{1\sigma }}$bond and double bond has ${{1\sigma \; and\; 1\pi\; bond}}$ whereas triple bond have ${{1\sigma \; and\; 2\pi \;\ bonds}}$ in it which makes it strongest.
Complete step by step answer:
First, we will see the type of overlapping and shape of the hybridized orbitals.
\[{{sp}}\]: The \[{{sp}}\] hybridization occurs when one s-orbital and one p-orbital overlap each other head on to form two \[{{sp}}\] hybridized orbitals having linear shape. Here two p-orbitals are unhybridized so they form ${{2\pi }}$ bonds.

${{s}}{{{p}}^{{2}}}$: Here in this hybridization one s-orbital and two p-orbitals overlap to form ${{3 s}}{{{p}}^{{2}}}$ hybridized orbitals and here one p-orbital is unhybridized so that it can form ${{\pi }}$-bond between two carbon or between carbon and a heteroatom. So ${{s}}{{{p}}^{{2}}}$ hybridized orbital has ${{3\sigma orbitals and 1\pi orbital}}$ to make single and double bond. It has trigonal shape

${{s}}{{{p}}^{{3}}}$ : In this hybridization one s-orbital overlaps with three p-orbital to form three ${{s}}{{{p}}^{{3}}}$ orbitals. Here we don’t have any unhybridized p-orbital to form ${{ \pi }}$ bond only ${{4\sigma }}$ bonds are formed and structure of these hybridized orbitals are tetrahedron.

So now in structure carbon ${{1}}$ has ${{4\sigma\; bonds\; no \;\pi \; bond}}$ so the hybridization of carbon 1 is $sp^3$
When we see carbon ${{2}}$ we observe it has ${{3\sigma \; and \; 1\pi \;bond}}$ and has trigonal planar structure so this fits the condition for ${{s}}{{{p}}^{{2}}}$ hybridization.
Now last carbon ${{3}}$ we see it has ${{2\sigma \; and \;2\pi \; bonds}}$ in it and structure is also linear then the hybridization is sp
So, the correct answer is Option C .
Note: By knowing the hybridization of the carbon or any element in a molecule we can know the nature of bond and characteristic of it whether the bond is strong or weak or it makes the molecule electron donating or withdrawing group.
The triple bond is the strongest among the single , double and triple bond because single bond only have ${{1\sigma }}$bond and double bond has ${{1\sigma \; and\; 1\pi\; bond}}$ whereas triple bond have ${{1\sigma \; and\; 2\pi \;\ bonds}}$ in it which makes it strongest.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




