
How many (x) moles of HI consumed?
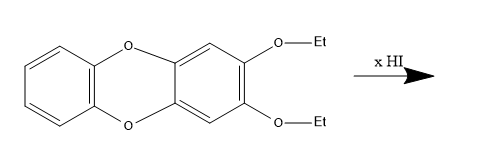

Answer
576k+ views
Hint: Ethers react with HI via nucleophilic substitution mechanism. Mechanism can be ${{S}_{N}}1$ or ${{S}_{N}}2$ depending upon the structure of alkyl groups in the molecule.
Complete step by step answer:
Let us first study the mechanism of nucleophilic substitution:
Nucleophilic substitution reaction-
Alkyl ethers are cleaved by the strong acids (HI or HBr); here HI, in nucleophilic substitution reaction (similar to that of alcohols).
The halide ions, bromide or iodide are both good nucleophiles. Depending upon the position and structure of alkyl groups in the molecule, reaction mechanism is adoptive i.e. ${{S}_{N}}1$ or ${{S}_{N}}2$.
Illustration-
Acidic cleavage of ethers-
When ethers are treated with strong acid in presence of a nucleophile, they can be cleaved to give alcohols and alkyl halides as the products.
As, in our case, if the ether is on primary carbon, ${{S}_{N}}2$ pathway is considered.
Mechanism-
1. HI protonated the ether oxygen, which turns into a better leaving group.
2. Iodide attacks the carbon by ${{S}_{N}}2$ mechanism.
Acidic cleavage example-
$R-O-R+HI\to R-O-H+RI$
Key point- Common acids for this process are hydrogen halides but sometimes ${{H}_{2}}S{{O}_{4}}$ in presence of ${{H}_{2}}O$ can be used.
According to the given reaction,
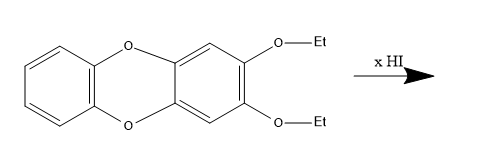
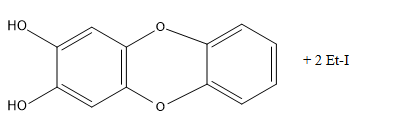
2 moles of HI are consumed in the reaction.
Note: Ether bond between two phenyl rings does not cleave as they do not result in stable carbocations.
Generally. HCl acid is not used for the nucleophilic substitution reaction as $C{{l}^{-}}$ is weak nucleophile and hence, reaction does not occur easily.
Complete step by step answer:
Let us first study the mechanism of nucleophilic substitution:
Nucleophilic substitution reaction-
Alkyl ethers are cleaved by the strong acids (HI or HBr); here HI, in nucleophilic substitution reaction (similar to that of alcohols).
The halide ions, bromide or iodide are both good nucleophiles. Depending upon the position and structure of alkyl groups in the molecule, reaction mechanism is adoptive i.e. ${{S}_{N}}1$ or ${{S}_{N}}2$.
Illustration-
Acidic cleavage of ethers-
When ethers are treated with strong acid in presence of a nucleophile, they can be cleaved to give alcohols and alkyl halides as the products.
As, in our case, if the ether is on primary carbon, ${{S}_{N}}2$ pathway is considered.
Mechanism-
1. HI protonated the ether oxygen, which turns into a better leaving group.
2. Iodide attacks the carbon by ${{S}_{N}}2$ mechanism.
Acidic cleavage example-
$R-O-R+HI\to R-O-H+RI$
Key point- Common acids for this process are hydrogen halides but sometimes ${{H}_{2}}S{{O}_{4}}$ in presence of ${{H}_{2}}O$ can be used.
According to the given reaction,


2 moles of HI are consumed in the reaction.
Note: Ether bond between two phenyl rings does not cleave as they do not result in stable carbocations.
Generally. HCl acid is not used for the nucleophilic substitution reaction as $C{{l}^{-}}$ is weak nucleophile and hence, reaction does not occur easily.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




