
How do you draw aromatic rings?
Answer
552k+ views
Hint:If we try to recall that molecular formula describes the exact number of atoms in a molecule as well as in the molecular formula of a benzene they consist of $6$ carbon atoms as well as it form a ring -like structure. Also ethane is the second member of an alkane series. Now by using these we all can easily draw aromatic rings.
Complete step-by-step answer:An aromatic ring is drawn with six sides and which is close in structure like naphthalene. Then we need to have compound with six carbons atoms also the some automatic rings are given as:
Naphthalene : It is an aromatic polycyclic hydrogen carbon compound which consist of a two benzene ring as well as have chemical formulae such as \[{{C}_{10}}{{H}_{8}}\] it also has molecular mass as $128$ It is still produced by distillation of a petrol as its white in color as well as volatile in a nature and has strong odor. The structure of naphthalene is given by:
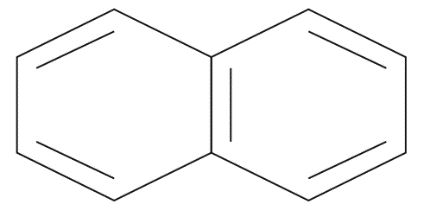
Methyl benzene: From the name benzene it’s getting clear that it’s an aromatic compound which consists of a cyclic ring that is a benzene ring as well as an alkyl group that is a methyl group. The carbon atoms of a benzene replace hydrogen atoms with methyl groups along with the formation of methyl benzene. It is given by chemical formula as \[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\] also its IUPAC name is given as Toluene. The structure of methyl benzene is given by;
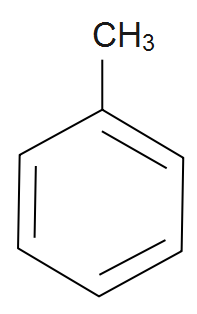
Note:It should be remembered that benzene is an aromatic compound and whenever ethyne gas is passed through a red hot tube then benzene is formed. Also we should remember that benzene can be also use as a non-polar solvent of large aromatic compound or we can say that organometallic complex
Complete step-by-step answer:An aromatic ring is drawn with six sides and which is close in structure like naphthalene. Then we need to have compound with six carbons atoms also the some automatic rings are given as:
Naphthalene : It is an aromatic polycyclic hydrogen carbon compound which consist of a two benzene ring as well as have chemical formulae such as \[{{C}_{10}}{{H}_{8}}\] it also has molecular mass as $128$ It is still produced by distillation of a petrol as its white in color as well as volatile in a nature and has strong odor. The structure of naphthalene is given by:

Methyl benzene: From the name benzene it’s getting clear that it’s an aromatic compound which consists of a cyclic ring that is a benzene ring as well as an alkyl group that is a methyl group. The carbon atoms of a benzene replace hydrogen atoms with methyl groups along with the formation of methyl benzene. It is given by chemical formula as \[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\] also its IUPAC name is given as Toluene. The structure of methyl benzene is given by;

Note:It should be remembered that benzene is an aromatic compound and whenever ethyne gas is passed through a red hot tube then benzene is formed. Also we should remember that benzene can be also use as a non-polar solvent of large aromatic compound or we can say that organometallic complex
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




