
How do you alkylate benzene?
Answer
548.4k+ views
Hint: Friedel Crafts reactions, similar to their biochemical counterparts, require reactive electrophiles with huge carbocation character. Perhaps the most widely recognized approaches to alkylate an aromatic ring is to utilize an alkyl chloride electrophile that is enacted by the expansion of aluminum or iron trichloride. The metal chloride fills in as a Lewis acid, tolerating electron density from the alkyl chloride. This has the effect of amplifying the carbon-chlorine dipole, making the carbon end more electropositive and along these lines more electrophilic – even to where the bond breaks and a particle pair is formed.
Complete step by step answer:
You can do it through a Friedel-Crafts Alkylation, where you have an alkyl halide and a solid Lewis acid catalyst. Generally we use \[Fe{X_3}\] where \[X\] is regularly \[Cl\] or \[Br\] .
The mechanism is as per the following, and is fundamentally the same as Friedel-Crafts Acylation (and even draws matches with the benzene halogenation reaction where you have\[{X_2}\] In \[Fe{X_3}\]...
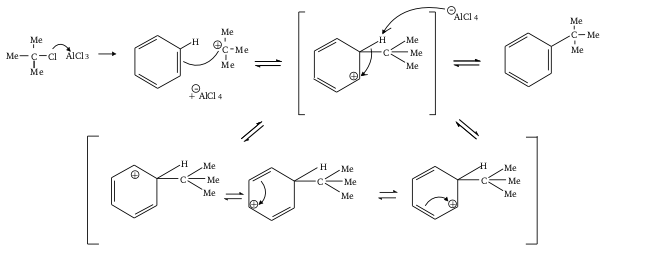
It is significant however that by adding an alkyl group onto benzene, you've added an actuating group; that is, the alkyl group gives electrons to the ring, making it more nucleophilic, and subsequently more inclined to responding in this Friedel-Crafts Alkylation.
Accordingly, you have a very pretty possibility for over alkylation in the event that you don't add a controlled measure of catalyst and reactant
Note: Aryl amines form highly unreactive complexes with lewis acid impetuses so we can't utilize them in this reaction.
The aromatic compound, which is less reactive than mono halobenzene, can't be utilized in this reaction.
Acylation reactions by and large form just ketones.
Complete step by step answer:
You can do it through a Friedel-Crafts Alkylation, where you have an alkyl halide and a solid Lewis acid catalyst. Generally we use \[Fe{X_3}\] where \[X\] is regularly \[Cl\] or \[Br\] .
The mechanism is as per the following, and is fundamentally the same as Friedel-Crafts Acylation (and even draws matches with the benzene halogenation reaction where you have\[{X_2}\] In \[Fe{X_3}\]...

It is significant however that by adding an alkyl group onto benzene, you've added an actuating group; that is, the alkyl group gives electrons to the ring, making it more nucleophilic, and subsequently more inclined to responding in this Friedel-Crafts Alkylation.
Accordingly, you have a very pretty possibility for over alkylation in the event that you don't add a controlled measure of catalyst and reactant
Note: Aryl amines form highly unreactive complexes with lewis acid impetuses so we can't utilize them in this reaction.
The aromatic compound, which is less reactive than mono halobenzene, can't be utilized in this reaction.
Acylation reactions by and large form just ketones.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

India is a sovereign socialist secular democratic republic class 12 social science CBSE

How many states of matter are there in total class 12 chemistry CBSE




