
How can the octet rule be violated?
Answer
558.9k+ views
Hint: Newland found out that every eighth element in the grouping resembles each other and put forward the law of octaves, in which we can see that he arranged all the elements known at that time in order of relative atomic mass into periodic table.
Complete step by step answer:
- Newland observed that when he arranged lighter elements in order of their increasing atomic masses, then every eighth element was the same as that of the first one just like the eighth note of a musical scale.
- And it is found that this relation is called as Newland’s law of octaves
It is found that octet rule is violated whenever a bonded atom has lower or greater than eight valence electrons in its valence shell.
We can see some of the examples:
- Incomplete octet:
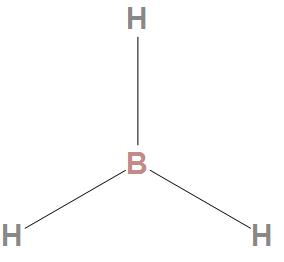
It is found that it has only six valence electrons around boron. And the boron atom has an incomplete octet.
- Odd-electron molecules:
It is found that nitrogen monoxide is having 11 valence electrons. And as we know that there is no way that both the atoms can get octet. We can see here that one electron always has 7 electrons in its valence shell.
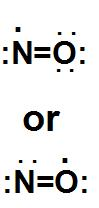
- Expanded octets: It is found that in the periodic table the non-metals present after silicon can expand their octets. And can have more than eight valence electrons around the central atom.
Note: - This law was found to be applicable only till calcium, after that every eighth element doesn’t match to the first element.
- As the noble gases were discovered, the properties of the 8th element were no longer found to be the same as those of the first one.
Complete step by step answer:
- Newland observed that when he arranged lighter elements in order of their increasing atomic masses, then every eighth element was the same as that of the first one just like the eighth note of a musical scale.
- And it is found that this relation is called as Newland’s law of octaves
It is found that octet rule is violated whenever a bonded atom has lower or greater than eight valence electrons in its valence shell.
We can see some of the examples:
- Incomplete octet:
It is found that it has only six valence electrons around boron. And the boron atom has an incomplete octet.

- Odd-electron molecules:
It is found that nitrogen monoxide is having 11 valence electrons. And as we know that there is no way that both the atoms can get octet. We can see here that one electron always has 7 electrons in its valence shell.
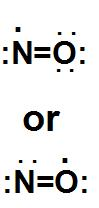
- Expanded octets: It is found that in the periodic table the non-metals present after silicon can expand their octets. And can have more than eight valence electrons around the central atom.
Note: - This law was found to be applicable only till calcium, after that every eighth element doesn’t match to the first element.
- As the noble gases were discovered, the properties of the 8th element were no longer found to be the same as those of the first one.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




