
How can I make an atomic model?
Answer
558.3k+ views
Hint: Protons, neutrons, and electrons are the subatomic particles of an atom. To calculate the number of protons and neutrons present in an atom, atomic number and atomic mass number are required. For the atomic mass and an atomic number of an refer to the periodic table.
The atomic model is used to determine the structure of the atom of an element, how the electrons, protons, and neutrons are placed in the atom.
Formula used:
\[Atomic\,number\, = \,number of protons or number of electrons\]
\[Atomic\,mass\, = \,number of protons + number of neutrons\]
Complete step-by-step answer:As we know that an atom contains a central nucleus. It is composed of particles that are protons and neutrons. Protons are positively charged while neutrons are neutral particles.Hence, the nucleus of an atom is positively charged.The electrons are present around the nucleus in shells and are negatively charged. In the case of the neutral atom number of the protons and the numbers of the electrons are equal hence neutral atom is charged less.Here, we consider the atom of lithium. The atomic number of the lithium is three and the mass number is seven. There are three electrons and protons present in the lithium and four neutrons are there in the lithium.Thus, the nucleus of lithium contains three protons and four neutrons. The three electrons are present around the nucleus.The diagrammatic representation of the lithium atom is as follows:
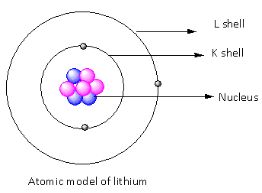
Here, in the diagram, pink balls represent the neutrons, blue balls represent the protons and black balls are the electrons.This is the atomic model of lithium. In this way, we can determine the atomic model.
Note:The mass number is the sum of the numbers of the protons and the numbers of the neutrons present in the nucleus.The mass of the electrons is negligible in comparison to the mass of the electrons hence, in the calculation of the mass number electrons are not involved.Electrons are negatively charged and protons are positively charged. The neutrons are chargeless particles.
The atomic model is used to determine the structure of the atom of an element, how the electrons, protons, and neutrons are placed in the atom.
Formula used:
\[Atomic\,number\, = \,number of protons or number of electrons\]
\[Atomic\,mass\, = \,number of protons + number of neutrons\]
Complete step-by-step answer:As we know that an atom contains a central nucleus. It is composed of particles that are protons and neutrons. Protons are positively charged while neutrons are neutral particles.Hence, the nucleus of an atom is positively charged.The electrons are present around the nucleus in shells and are negatively charged. In the case of the neutral atom number of the protons and the numbers of the electrons are equal hence neutral atom is charged less.Here, we consider the atom of lithium. The atomic number of the lithium is three and the mass number is seven. There are three electrons and protons present in the lithium and four neutrons are there in the lithium.Thus, the nucleus of lithium contains three protons and four neutrons. The three electrons are present around the nucleus.The diagrammatic representation of the lithium atom is as follows:

Here, in the diagram, pink balls represent the neutrons, blue balls represent the protons and black balls are the electrons.This is the atomic model of lithium. In this way, we can determine the atomic model.
Note:The mass number is the sum of the numbers of the protons and the numbers of the neutrons present in the nucleus.The mass of the electrons is negligible in comparison to the mass of the electrons hence, in the calculation of the mass number electrons are not involved.Electrons are negatively charged and protons are positively charged. The neutrons are chargeless particles.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




