
How can I draw metallic bonds?
Answer
558.6k+ views
Hint: Metals form cations and lose out the electrons which become delocalised and now a force of attraction is felt between the two opposite charges.
Complete step by step answer:
- In order to answer this question, we need to study the different types of bond in chemistry. Now, what are bonds? Bonds in chemistry show the connection between two atoms in a molecule. This can be strong or weak. So bond can be said as a force, or a link between atoms in a molecule or compound, that are neighbouring.
- Now, let us discuss the different types of bonds in chemistry. Firstly, we have ionic bonds. Now, we know that non metals are electronegative and metals are electropositive. So, the metals form positively charged cation and anions form negatively charged anion. The bond that is created between these two species is called an ionic bond and generally it is very strong.
- After ionic bond we have covalent bond and in this type of bond, the bond is formed by sharing of electrons. The sharing takes place in such a way that both the atoms satisfy the octet. This occurs mainly between non metals.
- Now let us come to metallic bonding. The bonds that metal ions in a metal make are called the metallic bonds. Metals tend to form cations and lose out the electrons. Now, the electrons become delocalised and this creates an attractive force between the metal ions and electrons. Following illustrates the metallic bonds in a metal:
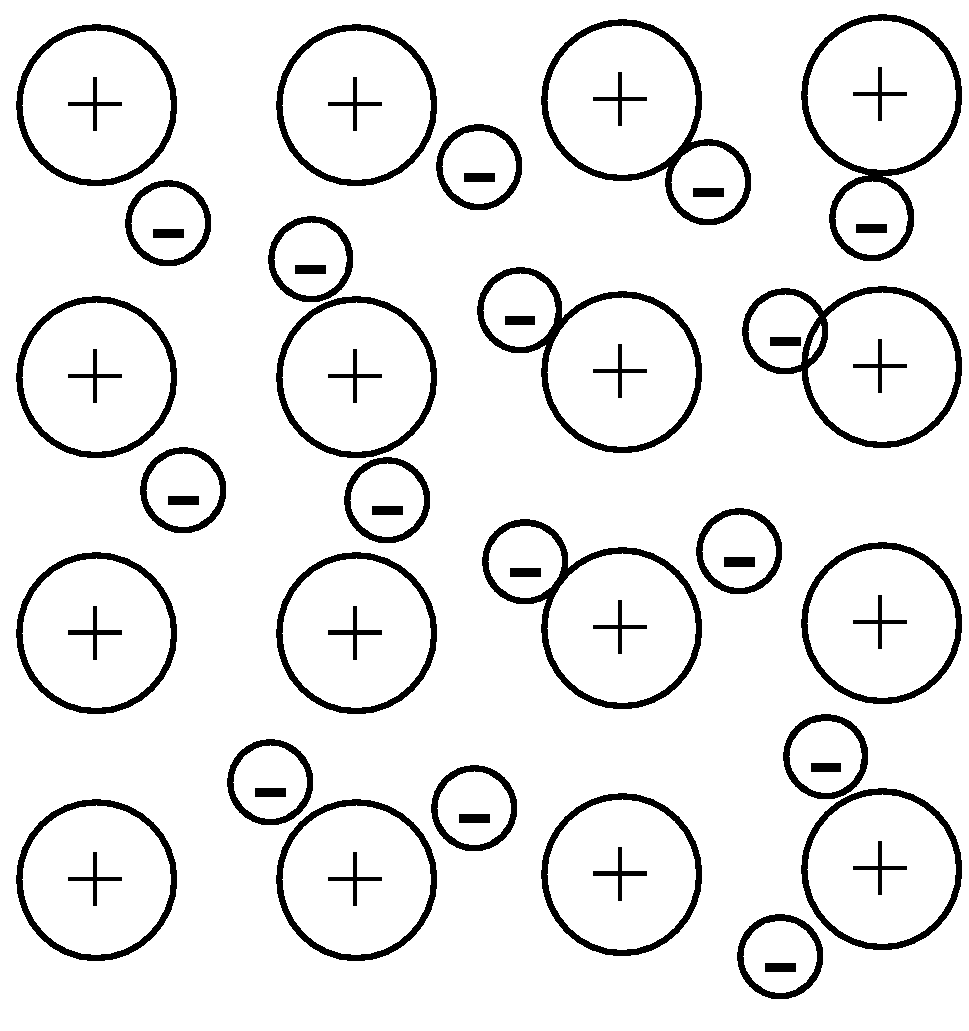
Note: Metallic bonds are strong but the strongest type of bond in chemistry is hydrogen bonding. It is formed when hydrogen makes a bond with an electronegative element like oxygen, fluorine or nitrogen. For example, water contains hydrogen bonding.
Complete step by step answer:
- In order to answer this question, we need to study the different types of bond in chemistry. Now, what are bonds? Bonds in chemistry show the connection between two atoms in a molecule. This can be strong or weak. So bond can be said as a force, or a link between atoms in a molecule or compound, that are neighbouring.
- Now, let us discuss the different types of bonds in chemistry. Firstly, we have ionic bonds. Now, we know that non metals are electronegative and metals are electropositive. So, the metals form positively charged cation and anions form negatively charged anion. The bond that is created between these two species is called an ionic bond and generally it is very strong.
- After ionic bond we have covalent bond and in this type of bond, the bond is formed by sharing of electrons. The sharing takes place in such a way that both the atoms satisfy the octet. This occurs mainly between non metals.
- Now let us come to metallic bonding. The bonds that metal ions in a metal make are called the metallic bonds. Metals tend to form cations and lose out the electrons. Now, the electrons become delocalised and this creates an attractive force between the metal ions and electrons. Following illustrates the metallic bonds in a metal:

Note: Metallic bonds are strong but the strongest type of bond in chemistry is hydrogen bonding. It is formed when hydrogen makes a bond with an electronegative element like oxygen, fluorine or nitrogen. For example, water contains hydrogen bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




