
Hinsberg reagent is used to distinguish between:
A. $-CHO,\text{ }>C=O$
B. $-C{{H}_{2}}OH,\text{ }>CHOH,\text{ }\to C-OH$
C. $-O-,\text{ }-OH$
D. $-N{{H}_{2}},\text{ }-NH-,\text{ }\to N$
Answer
588.6k+ views
Hint: Hinsberg’s reagent is another name for benzene sulfonyl chloride and its reaction with primary amines gives sulphonamide products that are soluble in alkalis. With this in mind, try to solve the given question.
Complete step by step answer:
Let us first analyse what Hinsberg’s reagent is as a chemical compound before moving onto its specific test where it is used to distinguish between a certain group of compounds.
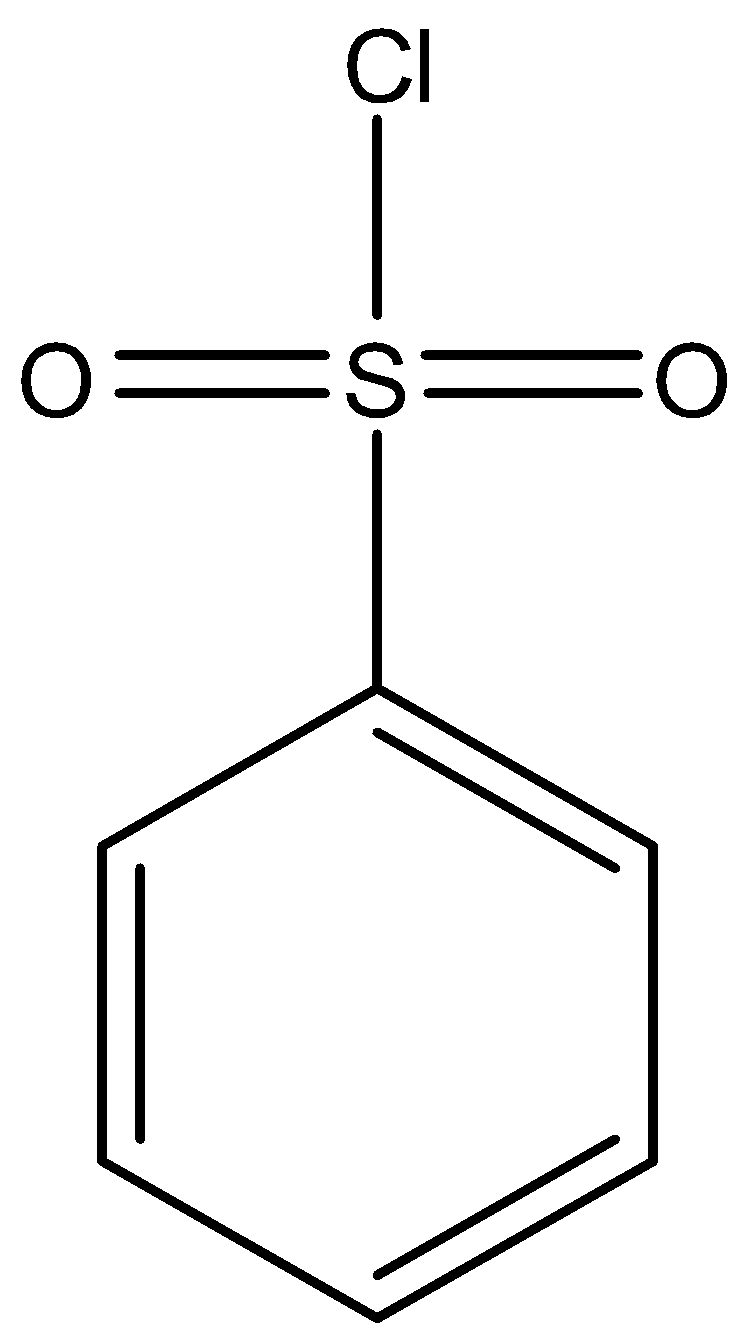
Hinsberg reagent is an alternative name for benzene sulfonyl chloride. This reagent is an organosulfur compound. Its chemical formula can be written as ${{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl$. This reagent looks like a viscous and colourless oil that dissolves in organic solvents due to its own organic nature.
Let us now observe the Hinsberg’s reagent test and its properties.
The Hinsberg reaction is a test for the detection of primary, secondary and tertiary amines. In this test, the reagent along with the amine is added to the solution of an aqueous alkali, like $NaOH$ or $KOH$, and shaken vigorously to carry out the reaction. A reagent containing an aqueous sodium hydroxide solution and benzenesulfonyl chloride is added to the concerned amine. A primary amine will form a soluble sulphonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulphonamide. A tertiary amine will not react with the sulfonamide but is insoluble in the solution containing the Hinsberg reagent and the aqueous alkali.
Let us observe the Hinsberg reagent’s reaction with different degrees of amine.
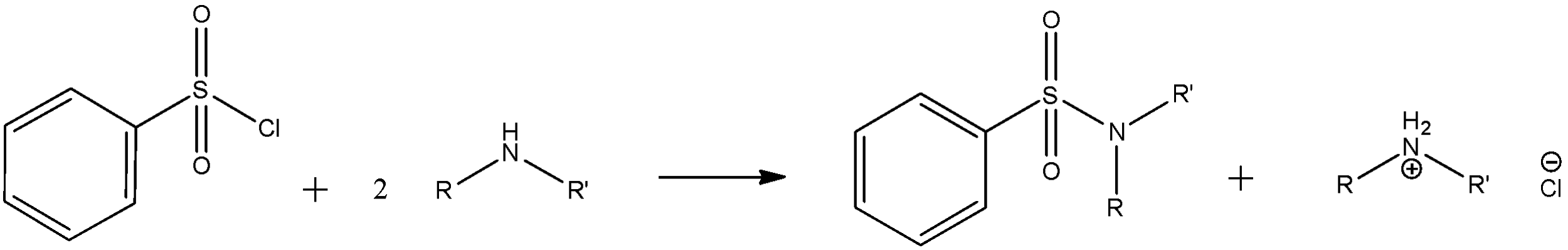
Amines serve as nucleophiles in attacking the sulfonyl chloride electrophile, displacing chloride. The sulphonamides resulting from primary and secondary amines are poorly soluble and precipitate as solids from solution:
\[PhS{{O}_{2}}Cl\text{ }+\text{ }2\text{ }RR'NH\text{ }\to \text{ }PhS{{O}_{2}}NRR'\text{ }+\text{ }[RR'N{{H}_{2}}^{+}]Cl\]
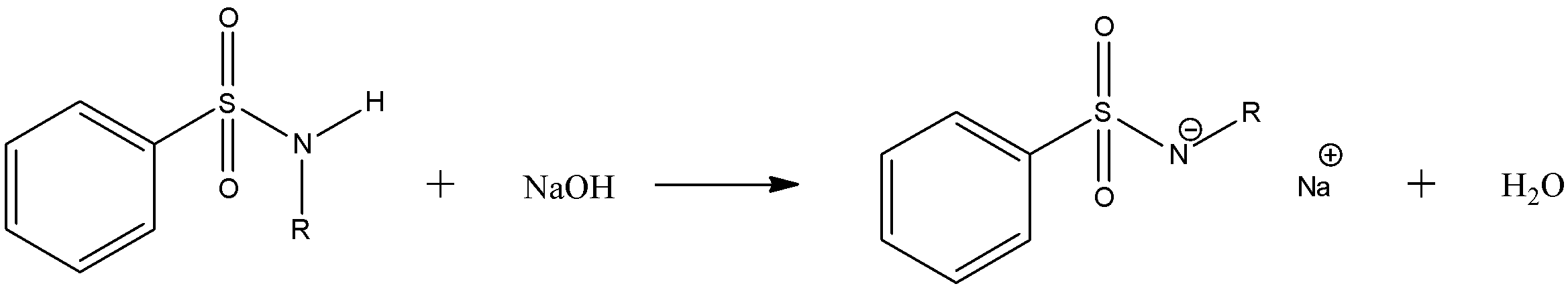
For primary amines (R' = H), the initially formed sulphonamide is deprotonated by base to give water-soluble sulphonamide salt ($N{{a}^{+}}[PhS{{O}_{2}}N{{R}^{-}}]$):
\[PhS{{O}_{2}}N\left( H \right)R\text{ }+\text{ }NaOH\text{ }\to \text{ }N{{a}^{+}}[PhS{{O}_{2}}N{{R}^{-}}]\text{ }+\text{ }{{H}_{2}}O\]
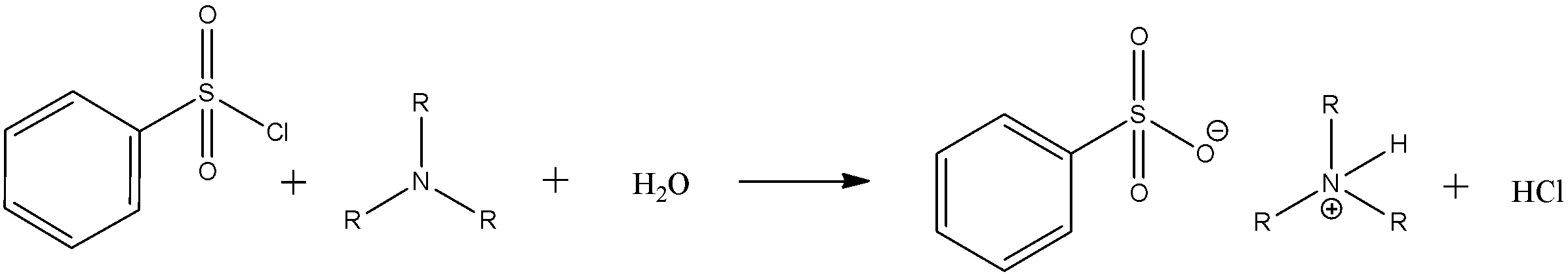
Tertiary amines promote hydrolysis of the sulfonyl chloride functional group, which affords water-soluble sulfonate salts.
\[PhS{{O}_{2}}Cl\text{ }+\text{ }{{R}_{3}}N\text{ }+\text{ }{{H}_{2}}O\text{ }\to \text{ }{{R}_{3}}N{{H}^{+}}[PhS{{O}_{3}}^{-}]\text{ }+\text{ }HCl\]
Thus, we can conclude that the answer to this question is ‘D. $-N{{H}_{2}},\text{ }-NH-,\text{ }\to N$
So, the correct answer is “Option D”.
Note: Tertiary amines are able to react with benzenesulfonyl chloride under a variety of conditions; the test described above is not absolute. The Hinsberg test for amines is valid only when reaction speed, concentration, temperature, and solubility are taken into account.
Complete step by step answer:
Let us first analyse what Hinsberg’s reagent is as a chemical compound before moving onto its specific test where it is used to distinguish between a certain group of compounds.
Hinsberg reagent is an alternative name for benzene sulfonyl chloride. This reagent is an organosulfur compound. Its chemical formula can be written as ${{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl$. This reagent looks like a viscous and colourless oil that dissolves in organic solvents due to its own organic nature.

Let us now observe the Hinsberg’s reagent test and its properties.
The Hinsberg reaction is a test for the detection of primary, secondary and tertiary amines. In this test, the reagent along with the amine is added to the solution of an aqueous alkali, like $NaOH$ or $KOH$, and shaken vigorously to carry out the reaction. A reagent containing an aqueous sodium hydroxide solution and benzenesulfonyl chloride is added to the concerned amine. A primary amine will form a soluble sulphonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulphonamide. A tertiary amine will not react with the sulfonamide but is insoluble in the solution containing the Hinsberg reagent and the aqueous alkali.
Let us observe the Hinsberg reagent’s reaction with different degrees of amine.
Amines serve as nucleophiles in attacking the sulfonyl chloride electrophile, displacing chloride. The sulphonamides resulting from primary and secondary amines are poorly soluble and precipitate as solids from solution:
\[PhS{{O}_{2}}Cl\text{ }+\text{ }2\text{ }RR'NH\text{ }\to \text{ }PhS{{O}_{2}}NRR'\text{ }+\text{ }[RR'N{{H}_{2}}^{+}]Cl\]

For primary amines (R' = H), the initially formed sulphonamide is deprotonated by base to give water-soluble sulphonamide salt ($N{{a}^{+}}[PhS{{O}_{2}}N{{R}^{-}}]$):
\[PhS{{O}_{2}}N\left( H \right)R\text{ }+\text{ }NaOH\text{ }\to \text{ }N{{a}^{+}}[PhS{{O}_{2}}N{{R}^{-}}]\text{ }+\text{ }{{H}_{2}}O\]

Tertiary amines promote hydrolysis of the sulfonyl chloride functional group, which affords water-soluble sulfonate salts.
\[PhS{{O}_{2}}Cl\text{ }+\text{ }{{R}_{3}}N\text{ }+\text{ }{{H}_{2}}O\text{ }\to \text{ }{{R}_{3}}N{{H}^{+}}[PhS{{O}_{3}}^{-}]\text{ }+\text{ }HCl\]

Thus, we can conclude that the answer to this question is ‘D. $-N{{H}_{2}},\text{ }-NH-,\text{ }\to N$
So, the correct answer is “Option D”.
Note: Tertiary amines are able to react with benzenesulfonyl chloride under a variety of conditions; the test described above is not absolute. The Hinsberg test for amines is valid only when reaction speed, concentration, temperature, and solubility are taken into account.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




