
Highest bond length will be present in the following:
(A)
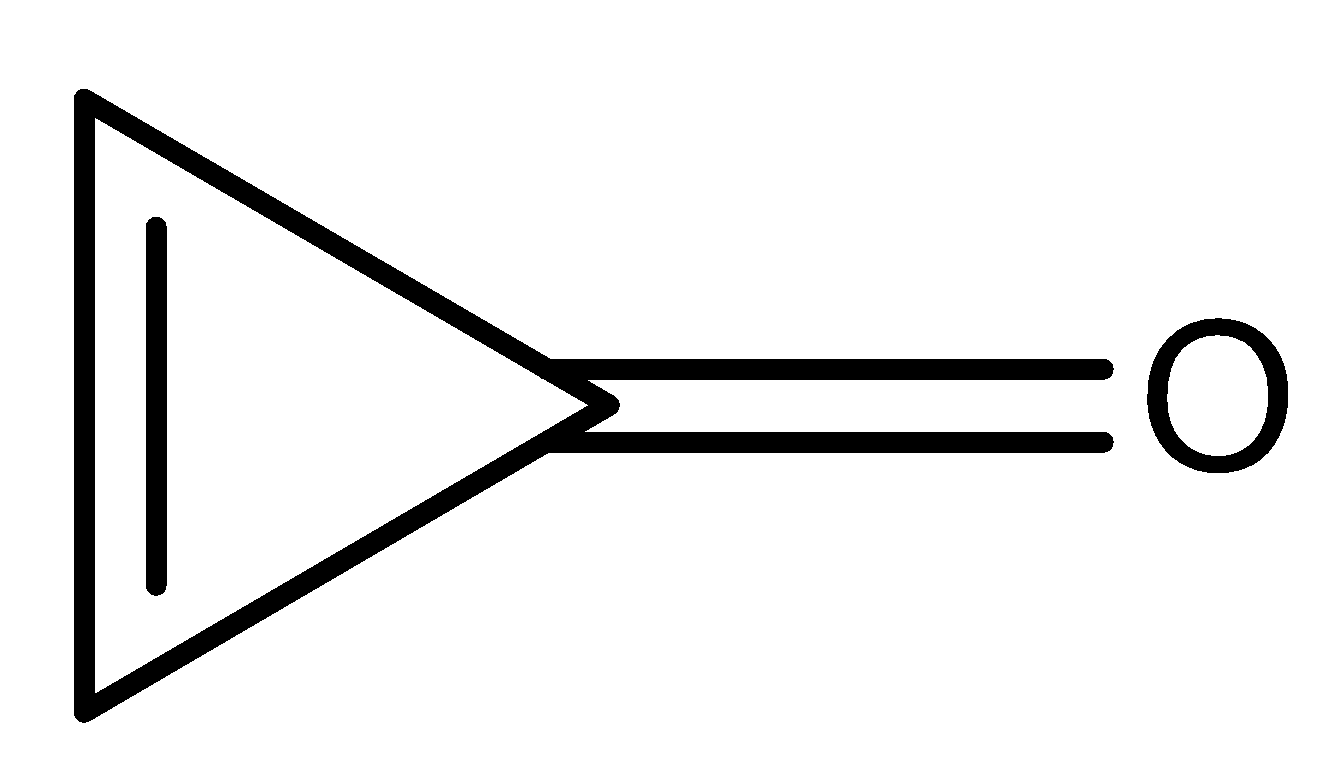
(B)
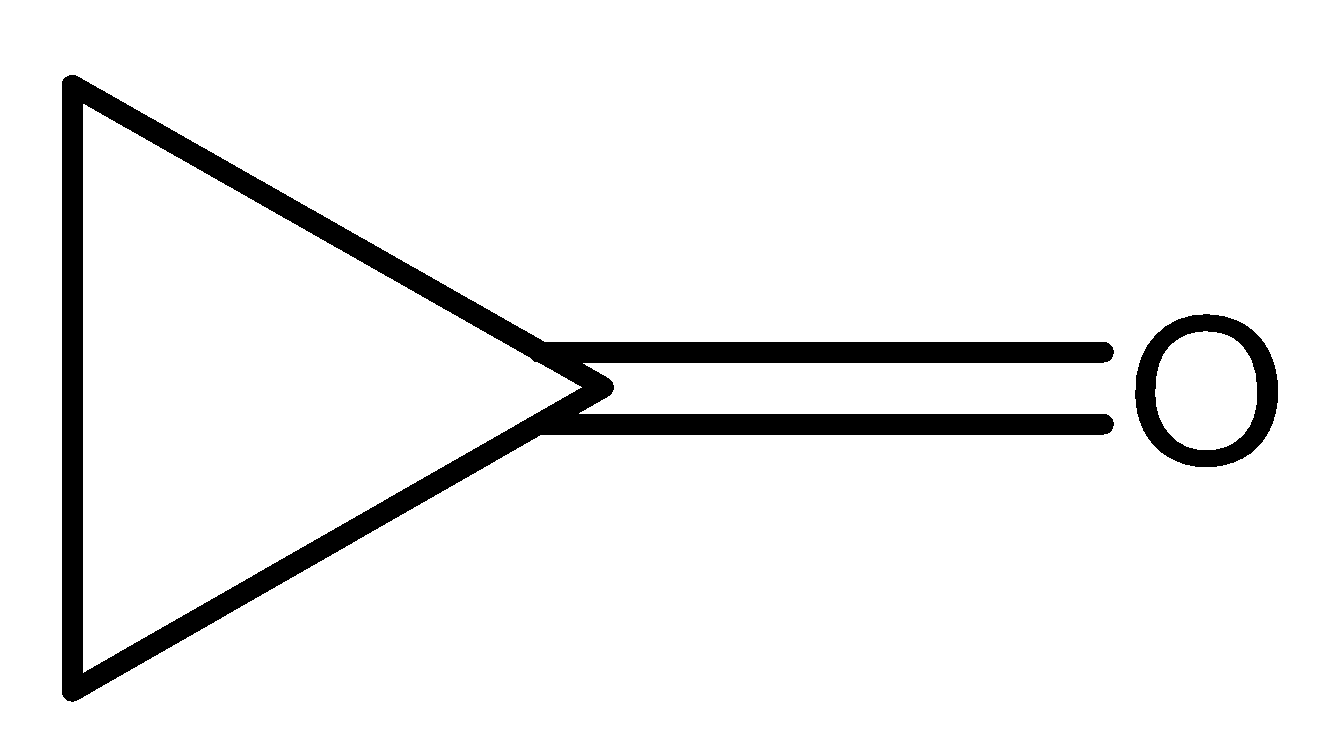
(C)
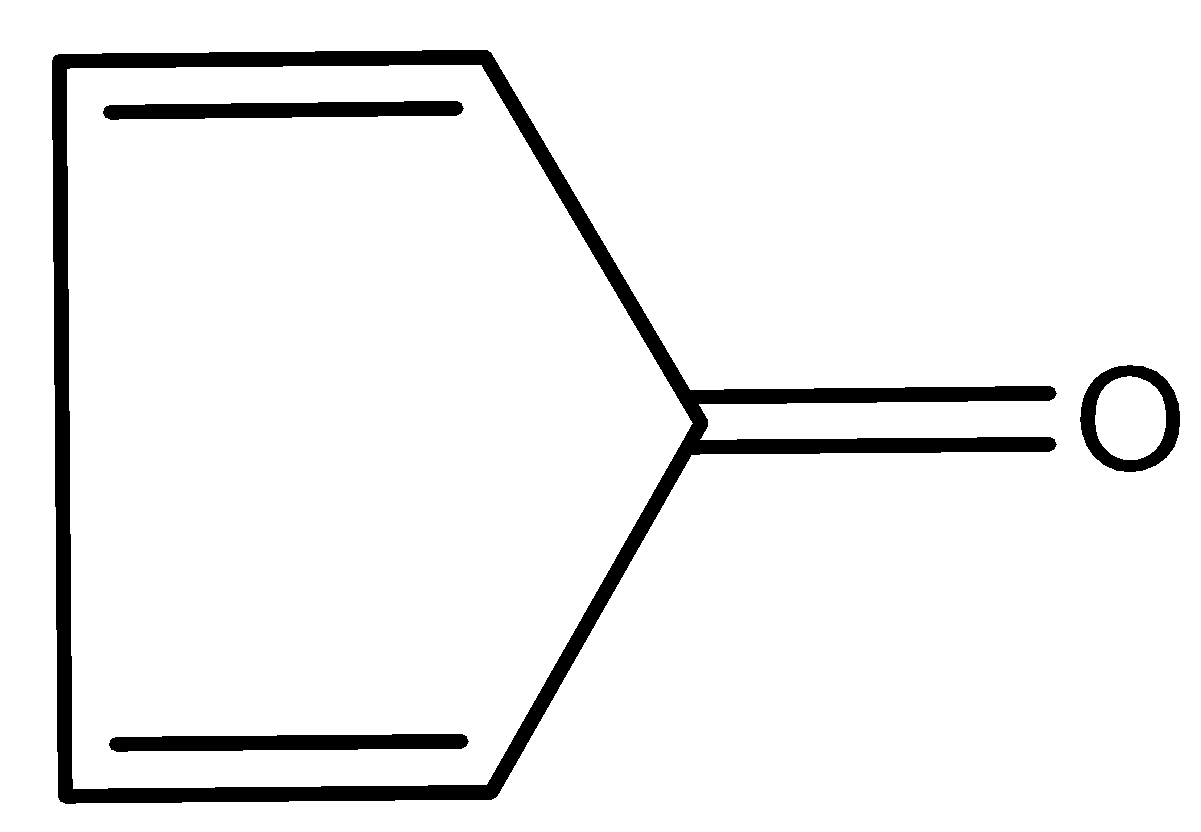
(D)
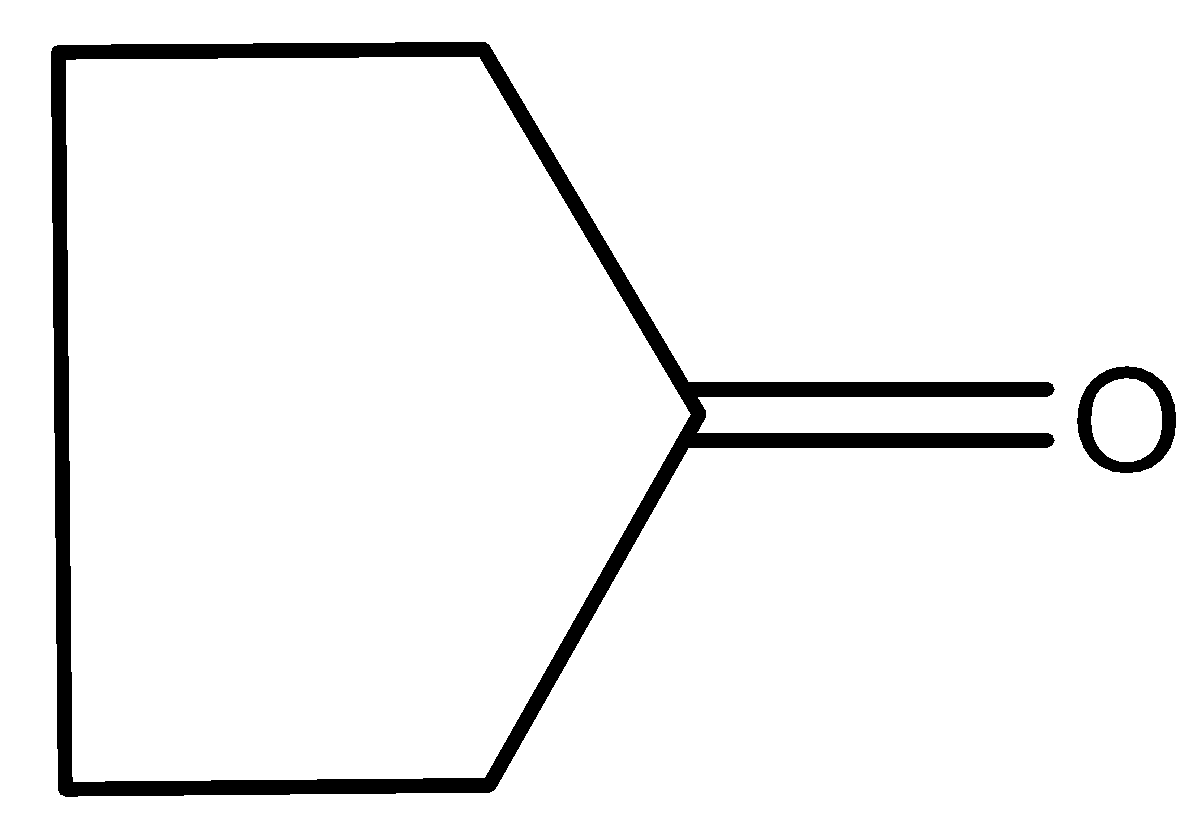




Answer
579.6k+ views
Hint: You should know that a single bond always has greater bond length than a double bond. Break the carbon-oxygen bond of the carbonyl group present in the cyclic compounds given in options and check their relative stabilities. An aromatic compound contains $(4n + 2)\pi $ electrons while an antiaromatic compound contains $(4n)\pi $ electrons.
Complete answer:
Let us first break the carbon-oxygen bond of each compound given in options.
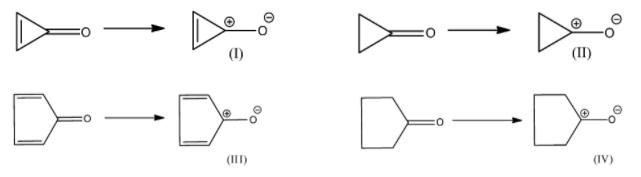
Oxygen being more electronegative than carbon, will acquire negative charge and carbon centre will have positive charge.
The compound (I) formed after breaking the carbonyl bond contains two $\pi $ electrons inside the cyclic ring. Hence it is aromatic because it follows Huckel’s rule of $(4n + 2)\pi $ electrons, where $n = 0$. Because of aromaticity, this compound formed is very stable. Hence, the compound (I) will try to remain in this form only. Thus, here a single bond character comes between carbon and oxygen atoms.
The compound (II) and (IV) have zero pi-electron in the ring. Thus, they are antiaromatic because they contain $(4n)\pi $ electrons, where $n = 0$. Due to anti-aromaticity, compound (II) and (IV) are unstable hence, will try to remain in the original form where the compound will have double-bond character in carbon and oxygen atoms.
The compound (III) has four pi-electrons in the ring. Hence, it also has $(4n)\pi $ electrons, where $n = 1$. Therefore, it is antiaromatic, unstable and will try to remain in the original form where the compound will have double-bond character in carbonyl bond.
Thus, we concluded that a single bond character is present only in compound (I) which is formed from the compound given in option A. In all other compounds, there is a double bond character between carbon-oxygen atoms. And, we all know that the bond length of a single bond is greater than that of a double bond. Hence, highest bond length will be present in the compound given in option A.
Thus, option A is correct.
Note:
The compound given in option (A) is a quasi-aromatic compound. These are those compounds in which the charges present on the molecule are a part of aromaticity of the compound.
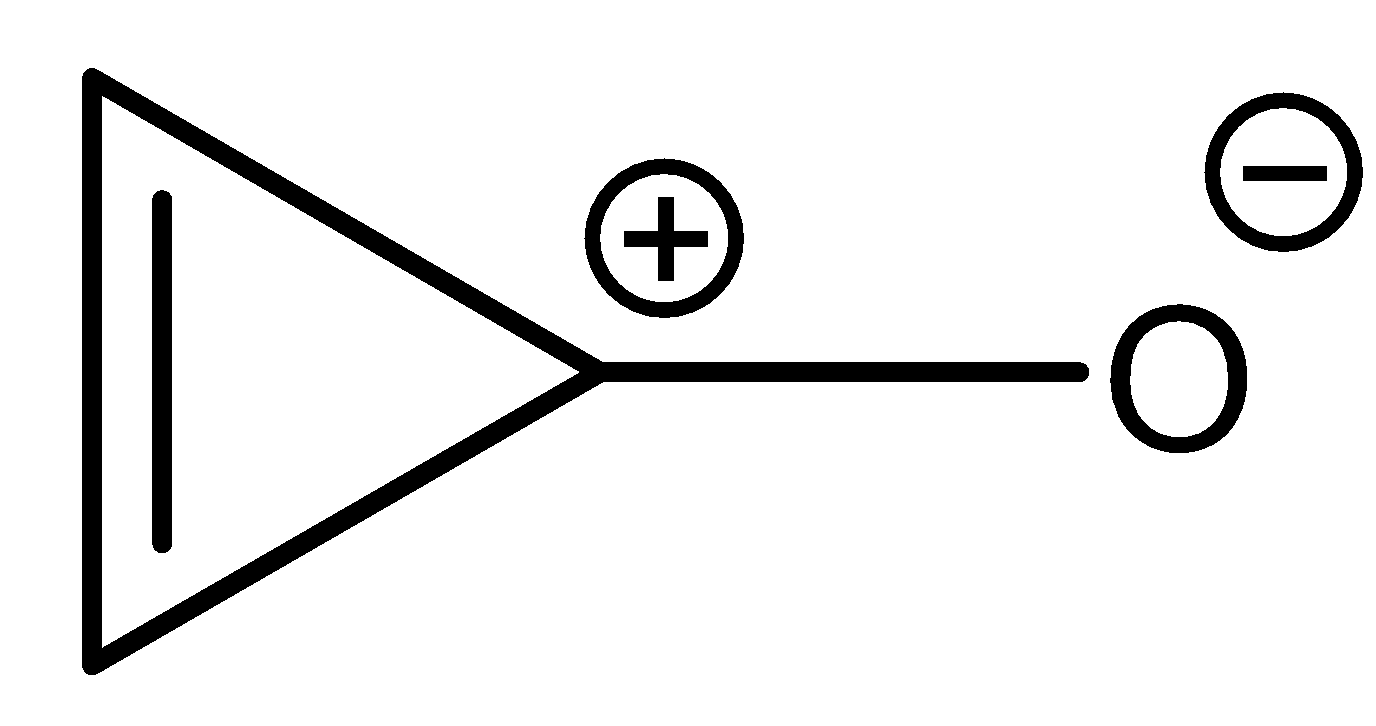
Here, pi-bond is in conjugation with the positive charge present on the molecule.
Complete answer:
Let us first break the carbon-oxygen bond of each compound given in options.

Oxygen being more electronegative than carbon, will acquire negative charge and carbon centre will have positive charge.
The compound (I) formed after breaking the carbonyl bond contains two $\pi $ electrons inside the cyclic ring. Hence it is aromatic because it follows Huckel’s rule of $(4n + 2)\pi $ electrons, where $n = 0$. Because of aromaticity, this compound formed is very stable. Hence, the compound (I) will try to remain in this form only. Thus, here a single bond character comes between carbon and oxygen atoms.
The compound (II) and (IV) have zero pi-electron in the ring. Thus, they are antiaromatic because they contain $(4n)\pi $ electrons, where $n = 0$. Due to anti-aromaticity, compound (II) and (IV) are unstable hence, will try to remain in the original form where the compound will have double-bond character in carbon and oxygen atoms.
The compound (III) has four pi-electrons in the ring. Hence, it also has $(4n)\pi $ electrons, where $n = 1$. Therefore, it is antiaromatic, unstable and will try to remain in the original form where the compound will have double-bond character in carbonyl bond.
Thus, we concluded that a single bond character is present only in compound (I) which is formed from the compound given in option A. In all other compounds, there is a double bond character between carbon-oxygen atoms. And, we all know that the bond length of a single bond is greater than that of a double bond. Hence, highest bond length will be present in the compound given in option A.
Thus, option A is correct.
Note:
The compound given in option (A) is a quasi-aromatic compound. These are those compounds in which the charges present on the molecule are a part of aromaticity of the compound.

Here, pi-bond is in conjugation with the positive charge present on the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




