
Here,N – O – N bond angle is maximum in:
A. $N{{O}_{2}}^{+}$
B. $N{{O}_{2}}$
C. $N{{O}_{3}}$
D. ${{N}_{2}}{{O}_{3}}$
Answer
493.2k+ views
Hint: Bond angle is the angle between two adjacent atoms. The valence electrons of an atom are distributed as bond and lone pairs on a covalent molecule. These bond and lone pairs affect the bond angle of any molecule. This is according to the VSEPR theory, that the bond and lone pair of electrons decide the geometry of atoms and hence the bond angles.
Complete answer:
The shapes of molecules tell us the bond angles between them. These shapes are identified using VSEPR theory that stands for valence shell electron pair repulsion theory. This theory suggests that a shape of a molecule is dependent on the valence pair of electrons in the atoms of that molecule. These valence electrons are distributed in the form of bond pairs and lone pairs. The interactions of these pairs are in the order, lone pair – lone pair > lone pair – bond pair > bond pair – bond pair. This states that lone pair lone pair interaction is more due to which the shape of the molecule distorts and the bond angle changes.
The shape of $N{{O}_{2}}^{+}$ is linear as the valence electrons are distributed as two double bond pairs, as
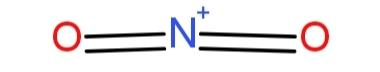 This linear arrangement of the molecule is due to the repulsions between the electron rich regions, so it has a bond angle of $180{}^\circ $.
This linear arrangement of the molecule is due to the repulsions between the electron rich regions, so it has a bond angle of $180{}^\circ $.
While,$N{{O}_{2}}$ structure
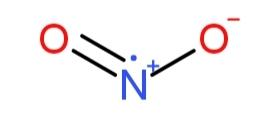 consists of an extra electron pair so, electron density due to the absence of 2 double bonds creates less repulsion, so it does not have maximum bond angle as the angle is $134{}^\circ $.
consists of an extra electron pair so, electron density due to the absence of 2 double bonds creates less repulsion, so it does not have maximum bond angle as the angle is $134{}^\circ $.
$N{{O}_{3}}$
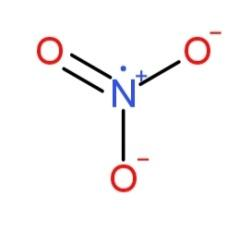 And ${{N}_{2}}{{O}_{3}}$
And ${{N}_{2}}{{O}_{3}}$
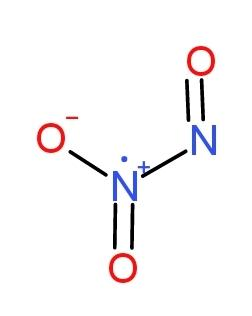 has bond angles of $120{}^\circ $ and $130{}^\circ $ respectively.
has bond angles of $120{}^\circ $ and $130{}^\circ $ respectively.
So, the N – O – N bond angle is maximum in $N{{O}_{2}}^{+}$.
Therefore option A is correct.
Note:
$N{{O}_{3}}$ has trigonal planar geometry that makes the angles 120 degree. The VSEPR theory suggests that the presence of lone pairs and bond pairs create repulsions to the extent that they change the shape of any molecule. The $N{{O}_{2}}^{+}$ molecule consist of one electron less on nitrogen due to which the double bonds are formed that makes the shape linear.
Complete answer:
The shapes of molecules tell us the bond angles between them. These shapes are identified using VSEPR theory that stands for valence shell electron pair repulsion theory. This theory suggests that a shape of a molecule is dependent on the valence pair of electrons in the atoms of that molecule. These valence electrons are distributed in the form of bond pairs and lone pairs. The interactions of these pairs are in the order, lone pair – lone pair > lone pair – bond pair > bond pair – bond pair. This states that lone pair lone pair interaction is more due to which the shape of the molecule distorts and the bond angle changes.
The shape of $N{{O}_{2}}^{+}$ is linear as the valence electrons are distributed as two double bond pairs, as
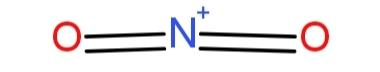
While,$N{{O}_{2}}$ structure

$N{{O}_{3}}$


So, the N – O – N bond angle is maximum in $N{{O}_{2}}^{+}$.
Therefore option A is correct.
Note:
$N{{O}_{3}}$ has trigonal planar geometry that makes the angles 120 degree. The VSEPR theory suggests that the presence of lone pairs and bond pairs create repulsions to the extent that they change the shape of any molecule. The $N{{O}_{2}}^{+}$ molecule consist of one electron less on nitrogen due to which the double bonds are formed that makes the shape linear.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




