
Heaviest naturally occurring element. Identify the unknown element detected by Hahn.
A. Barium-144
B. Barium-141
C. Barium-140
D.Xenon-141

Answer
571.8k+ views
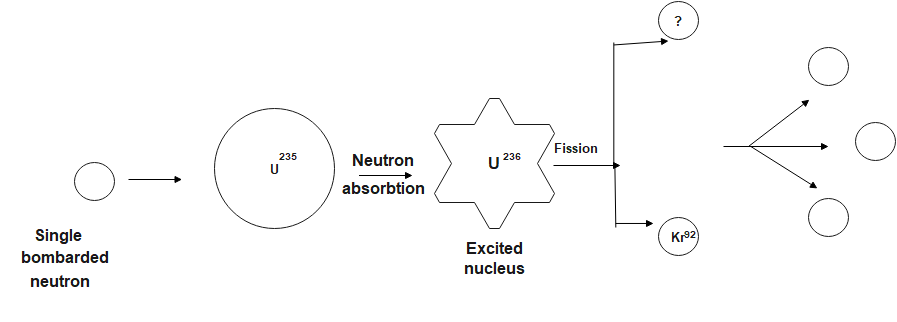
Hint: The original uranium-235 nucleus absorbs a neutron, splits into a nucleus of krypton-92 and a nucleus of barium-141, and upon splitting, releases three more neutrons. Those three neutrons can then cause three more nuclei of uranium-235 to fission, each of which releases more neutrons, and so on.
Complete answer:
in two extremely unstable fission fragments, a barium and a krypton nucleus, one of the many known fission reactions of uranium-235 induced by absorbing a neutron result. Almost instantaneously, these fragments release three neutrons between them, becoming barium-144 and krypton-89. In turn, by repeated beta decay, barium-144 is gradually converted to other fission products, lanthanum-144, cerium-144, praseodymium-144, and ultimately relatively stable neodymium-144; and krypton-89 is similarly transformed by rubidium-89 and strontium-89 into stable yttrium-89. The chemical properties and radioactive properties of fission products, such as their half-lives and the kinds of particles they emit, are identified by fission products.
The fission reaction is:
${ }^{236} \mathrm{U} \rightarrow{ }^{92 \mathrm{Kr}}+{ }^{\mathrm{A}} \mathrm{X}$
In a nuclear reaction, the net mass number of the reactant must be equal to that of the product. $\therefore 236=92+\mathrm{~A}$
$\text{A=144}$
Thus, the element detected: Barium – 144
The correct option is (A).
Additional Information:
Typically, elements such as krypton, strontium, caesium and barium are the fission products. The problem is that the radioactive isotopes of these elements are produced by nuclear fission. These radioactive isotopes will decay and give out ionizing radiation as they do so in the normal manner.
Note:
Due to their abnormally large number of neutrons compared to protons, the fission fragments are highly unstable; therefore, they undergo successive radioactive decays by emitting neutrons, converting neutrons into protons, antineutrinos, and ejected electrons (beta decay), and radiating energy (gamma decay).
Complete answer:
in two extremely unstable fission fragments, a barium and a krypton nucleus, one of the many known fission reactions of uranium-235 induced by absorbing a neutron result. Almost instantaneously, these fragments release three neutrons between them, becoming barium-144 and krypton-89. In turn, by repeated beta decay, barium-144 is gradually converted to other fission products, lanthanum-144, cerium-144, praseodymium-144, and ultimately relatively stable neodymium-144; and krypton-89 is similarly transformed by rubidium-89 and strontium-89 into stable yttrium-89. The chemical properties and radioactive properties of fission products, such as their half-lives and the kinds of particles they emit, are identified by fission products.
The fission reaction is:
${ }^{236} \mathrm{U} \rightarrow{ }^{92 \mathrm{Kr}}+{ }^{\mathrm{A}} \mathrm{X}$
In a nuclear reaction, the net mass number of the reactant must be equal to that of the product. $\therefore 236=92+\mathrm{~A}$
$\text{A=144}$
Thus, the element detected: Barium – 144
The correct option is (A).
Additional Information:
Typically, elements such as krypton, strontium, caesium and barium are the fission products. The problem is that the radioactive isotopes of these elements are produced by nuclear fission. These radioactive isotopes will decay and give out ionizing radiation as they do so in the normal manner.
Note:
Due to their abnormally large number of neutrons compared to protons, the fission fragments are highly unstable; therefore, they undergo successive radioactive decays by emitting neutrons, converting neutrons into protons, antineutrinos, and ejected electrons (beta decay), and radiating energy (gamma decay).
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




